Hot Lithography Vat Photopolymerisation 3D Printing: Vat Temperature vs. Mixture Design
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Mixture Preparation
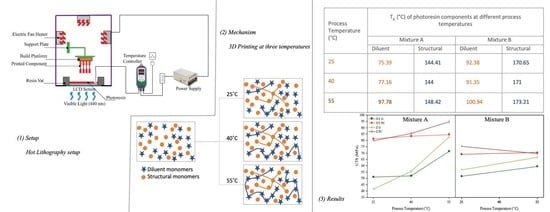
2.2. Hot Lithography 3D Printing
2.3. Viscosity Measurements
2.4. Depth of Cure Measurements
2.5. Degree of Conversion
2.6. Tensile Properties
2.7. Dynamic Mechanical Analysis (DMA)
2.8. Dimensional Studies
- Polymerisation shrinkage: For this purpose, the dimension test parts [23] were measured on the platform. The absolute dimensions of the as-printed parts on the platform are reported as an indication of their polymerisation shrinkage.
- Susceptibility to deformation: For this purpose, the same samples used in the previous step were measured before and after detachment from the build-platform while in the green state. The deviation (in percentage) is taken as an indication of the “accumulated internal residual stresses”, which are partially released upon removal from the platform [24] and therefore their susceptibility to deformation as a result of “stress relaxation” (in the green state).
- Dimensional stability: Same parts were aged in an oven and away from light at 25 °C for 7 days and measured again. The difference between the dimensions of the parts before and after the 7-day ageing period was taken as an indication of their dimensional stability (reported in percentage).
3. Results and Discussions
3.1. Depth of Cure Measurements
3.2. Degree of Conversion
3.3. Viscosity Measurements
3.4. Mechanical Properties
3.5. Dynamic Mechanical Analysis (DMA)
3.6. Dimensional Studies
- Polymerisation shrinkage
- 2.
- Susceptibility to deformation
- 3.
- Dimensional stability
4. Conclusions
- Hot lithography can significantly enhance the mechanical and dimensional properties of the parts when the right print temperature is chosen for a given mixture. Monomers with a lower network Tg (closer to the process temperature) respond more readily to the applied heat during hot lithography. In this case, hot lithography leads to an enhanced DC, hence improving the mechanical properties of the mixture printed at that temperature. This is particularly useful as aside from reducing the mixture’s viscosity, which in turns lowers the separation forces, higher mechanical properties of the prints reduce the chance of deformation caused by the remainder of the separation and peeling forces during printing.
- Hot lithography at the right temperature can significantly reduce the susceptibility of the parts to deformation, which occurs after the printing process (e.g., when detached from the build platform), and later when aged or post-cured. This is particularly useful for applications in which the dimensional accuracy is of great importance.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, J.; Xiao, P. 3D printing of photopolymers. Polym. Chem. 2018, 9, 1530–1540. [Google Scholar] [CrossRef]
- Gritsenko, D.; Ahmadian Yazdi, A.; Lin, Y.; Hovorka, V.; Pan, Y.; Xu, J. On characterization of separation force for resin replenishment enhancement in 3D printing. Addit. Manuf. 2017, 17, 151–156. [Google Scholar] [CrossRef]
- Song, H.; Rodriguez, N.A.; Seepersad, C.C.; Crawford, R.H.; Chen, M.; Duoss, E.B. Development of a variable tensioning system to reduce separation force in large scale stereolithography. Addit. Manuf. 2021, 38, 101816. [Google Scholar] [CrossRef]
- Pan, Y.; He, H.; Xu, J.; Feinerman, A. Study of separation force in constrained surface projection stereolithography. Rapid Prototyp. J. 2017, 23, 353–361. [Google Scholar] [CrossRef] [Green Version]
- Mendes-Felipe, C.; Patrocinio, D.; Laza, J.M.; Ruiz-Rubio, L.; Vilas-Vilela, J.L. Evaluation of postcuring process on the thermal and mechanical properties of the Clear02™ resin used in stereolithography. Polym. Test. 2018, 72, 115–121. [Google Scholar] [CrossRef]
- Tosto, C.; Pergolizzi, E.; Blanco, I.; Patti, A.; Holt, P.; Karmel, S.; Cicala, G. Epoxy Based Blends for Additive Manufacturing by Liquid Crystal Display (LCD) Printing: The Effect of Blending and Dual Curing on Daylight Curable Resins. Polymers 2020, 12, 1594. [Google Scholar] [CrossRef]
- Kowsari, K.; Zhang, B.; Panjwani, S.; Chen, Z.; Hingorani, H.; Akbari, S.; Fang, N.X.; Ge, Q. Photopolymer formulation to minimize feature size, surface roughness, and stair-stepping in digital light processing-based three-dimensional printing. Addit. Manuf. 2018, 24, 627–638. [Google Scholar] [CrossRef]
- Gibson, I.; Rosen, D.; Stucker, B. Vat Photopolymerization Processes. In Additive Manufacturing Technologies; Springer: New York, NY, USA, 2015; pp. 63–106. [Google Scholar] [CrossRef]
- Bagheri, A.; Jin, J. Photopolymerization in 3D Printing. ACS Appl. Polym. Mater. 2019, 1, 593–611. [Google Scholar] [CrossRef] [Green Version]
- Schricker, S.R. Composite Resin Polymerization and Relevant Parameters. In Orthodontic Applications of Biomaterials; Elsevier: Amsterdam, The Netherlands, 2017; pp. 153–170. [Google Scholar] [CrossRef]
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mülhaupt, R. Polymers for 3D Printing and Customized Additive Manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef] [Green Version]
- Dickens, S.H.; Stansbury, J.W.; Choi, K.M.; Floyd, C.J.E. Photopolymerization Kinetics of Methacrylate Dental Resins. Macromolecules 2003, 36, 6043–6053. [Google Scholar] [CrossRef]
- Tu, J.; Makarian, K.; Alvarez, N.J.; Palmese, G.R. Formulation of a Model Resin System for Benchmarking Processing-Property Relationships in High-Performance Photo 3D Printing Applications. Materials 2020, 13, 4109. [Google Scholar] [CrossRef] [PubMed]
- Marasinghe, L.; Croutxé-Barghorn, C.; Allonas, X.; Criqui, A. Effect of reactive monomers on polymer structure and abrasion resistance of UV cured thin films. Prog. Org. Coat. 2018, 118, 22–29. [Google Scholar] [CrossRef]
- Bartolo, P.; Mitchell, G. Stereo-thermal-lithography: A new principle for rapid prototyping. Rapid Prototyp. J. 2003, 9, 150–156. [Google Scholar] [CrossRef]
- Jongsma, L.A.; Kleverlaan, C.J. Influence of temperature on volumetric shrinkage and contraction stress of dental composites. Dent. Mater. 2015, 31, 721–725. [Google Scholar] [CrossRef]
- Stansbury, J.W. Dimethacrylate network formation and polymer property evolution as determined by the selection of monomers and curing conditions. Dent. Mater. 2012, 28, 13–22. [Google Scholar] [CrossRef] [Green Version]
- Steyrer, B.; Busetti, B.; Harakály, G.; Liska, R.; Stampfl, J. Hot Lithography vs. room temperature DLP 3D-printing of a dimethacrylate. Addit. Manuf. 2018, 21, 209–214. [Google Scholar] [CrossRef]
- Oezkan, B.; Sameni, F.; Karmel, S.; Engstrøm, D.S.; Sabet, E. A systematic study of vat-polymerization binders with potential use in the ceramic suspension 3D printing. Addit. Manuf. 2021, 47, 102225. [Google Scholar] [CrossRef]
- Kim, Y.C.; Hong, S.; Sun, H.; Kim, M.G.; Choi, K.; Cho, J.; Choi, H.R.; Koo, J.C.; Moon, H.; Byun, D.; et al. UV-curing kinetics and performance development of in situ curable 3D printing materials. Eur. Polym. J. 2017, 93, 140–147. [Google Scholar] [CrossRef]
- Pfaffinger, M. Hot Lithography—New Possibilities in Polymer 3D Printing. Laser Tech. J. 2018, 15, 45–47. [Google Scholar] [CrossRef] [Green Version]
- Dall’Argine, C.; Hochwallner, A.; Klikovits, N.; Liska, R.; Stampf, J.; Sangermano, M. Hot-Lithography SLA-3D Printing of Epoxy Resin. Macromol. Mater. Eng. 2020, 305, 2000325. [Google Scholar] [CrossRef]
- Weng, Z.; Zhou, Y.; Lin, W.; Senthil, T.; Wu, L. Structure-property relationship of nano enhanced stereolithography resin for desktop SLA 3D printer. Compos. Part A Appl. Sci. Manuf. 2016, 88, 234–242. [Google Scholar] [CrossRef]
- Pang, T.H.; Guertin, M.D.; Nguyen, H.D. Accuracy of Stereolithography Parts: Mechanism and Modes of Distortion for a ‘Letter-H’ Diagnostic Part. In Proceedings of the International Solid Freeform Fabrication Symposium, Austin, US, 1995; pp. 170–180. Available online: https://www.semanticscholar.org/paper/Accuracy-of-Stereolithography-Parts%3A-Mechanism-and-Pang-Guertin/086f117faee7538f05fa35ac5979c6e092151d76 (accessed on 25 June 2022).
- Yu, Q.; Nauman, S.; Santerre, J.P.; Zhu, S. Photopolymerization behavior of di(meth)acrylate oligomers. J. Mater. Sci. 2001, 36, 3599–3605. [Google Scholar] [CrossRef]
- Yang, Y.; Li, L.; Zhao, J. Mechanical property modeling of photosensitive liquid resin in stereolithography additive manufacturing: Bridging degree of cure with tensile strength and hardness. Mater. Des. 2018, 162, 418–428. [Google Scholar] [CrossRef]
- Hofstetter, C.; Orman, S.; Baudis, S.; Stampfl, J. Combining cure depth and cure degree, a new way to fully characterize novel photopolymers. Addit. Manuf. 2018, 24, 166–172. [Google Scholar] [CrossRef]
- Watts, D.; Alnazzawi, A. Temperature-dependent polymerization shrinkage stress kinetics of resin-composites. Dent. Mater. 2014, 30, 654–660. [Google Scholar] [CrossRef]
- Corder, R.D.; Dudick, S.C.; Bara, J.E.; Khan, S.A. Photorheology and Gelation during Polymerization of Coordinated Ionic Liquids. ACS Appl. Polym. Mater. 2020, 2, 2397–2405. [Google Scholar] [CrossRef]












| Monomer | Mw (g/mol) | Network Tg (°C) | Viscosity (mPa.s) * |
|---|---|---|---|
| Urethane dimethacrylate (UDMA) | 470 | 134 | 10,000 |
| Triethylene glycol dimethacrylate (TEGDMA) | 286 | 53 | 5–30 |
| Tris(2-hydroxyEthyl) isocyanurate triacrylate (THEICTA) | 423 | 240 | Crystalline solid |
| Dipropylene glycol diacrylate (DPGDA) | 242 | 104 | 5–15 |
| Monomer Content in the Mixtures (Total of 100 wt.%) | Other Additives on Top of 100 wt.% Monomer Mixture | |||||
|---|---|---|---|---|---|---|
| TEGDMA (wt.%) | UDMA (wt.%) | DPGDA (wt.%) | THEICTA (wt.%) | BAPO * | Black Pigment Stock * | |
| (wt.% on Top of the Monomer Mixtures) | ||||||
| Mixture A | 30 | 70 | - | - | 1 | 1.5 |
| Mixture B | - | - | 30 | 70 | 1 | 1.5 |
| Exposure Time (s) | Mixture A (µm) | Mixture B (µm) | |||||
|---|---|---|---|---|---|---|---|
| 25 (°C) | 40 (°C) | 55 (°C) | 25 (°C) | 40 (°C) | 55 (°C) | ||
| 3 * | 270 | 480 | 520 | 252 | 461 | 510 | |
| 10 | 230 | 250 | 260 | 200 | 220 | 250 | |
| 15 | 320 | 370 | 370 | 240 | 250 | 300 | |
| 20 | 410 | 420 | 440 | 300 | 330 | 390 | |
| Degree of Conversion (%) at Process Temperature | ||||
|---|---|---|---|---|
| 25 °C | 40 °C | 55 °C | 25 °C, Post-Cured | |
| Mixture A | 36.01 | 36.45 | 56.78 | 72.95 |
| Mixture B | 33.13 | 36.65 | 37.18 | 56.12 |
| Process Temperature (°C) | Glass Transition Temperature (°C) of Photoresin Components | |||
|---|---|---|---|---|
| Mixture A | Mixture B | |||
| TEGDMA | UDMA | DPGDA | THEICTA | |
| 25 | 75.39 | 144.41 | 92.38 | 170.65 |
| 40 | 77.16 | 144 | 91.35 | 171 |
| 55 | 97.78 | 148.42 | 100.94 | 173.21 |
| Post-cured | 106.55 | 144.01 | 117.17 | 174.53 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sameni, F.; Ozkan, B.; Zarezadeh, H.; Karmel, S.; Engstrøm, D.S.; Sabet, E. Hot Lithography Vat Photopolymerisation 3D Printing: Vat Temperature vs. Mixture Design. Polymers 2022, 14, 2988. https://doi.org/10.3390/polym14152988
Sameni F, Ozkan B, Zarezadeh H, Karmel S, Engstrøm DS, Sabet E. Hot Lithography Vat Photopolymerisation 3D Printing: Vat Temperature vs. Mixture Design. Polymers. 2022; 14(15):2988. https://doi.org/10.3390/polym14152988
Chicago/Turabian StyleSameni, Farzaneh, Basar Ozkan, Hanifeh Zarezadeh, Sarah Karmel, Daniel S. Engstrøm, and Ehsan Sabet. 2022. "Hot Lithography Vat Photopolymerisation 3D Printing: Vat Temperature vs. Mixture Design" Polymers 14, no. 15: 2988. https://doi.org/10.3390/polym14152988