A Theoretical Study on the Phosgenation of 2,4-Toluenediamine (2,4-TDA)
Abstract
:1. Introduction
2. Computational Methods
3. Results and Discussion
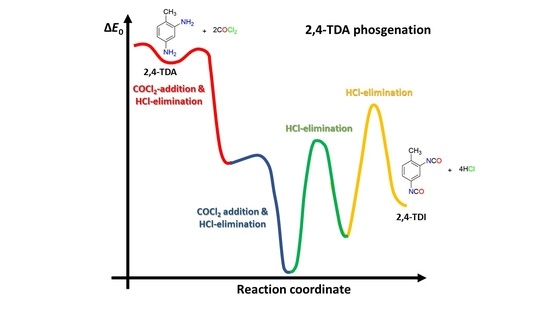
3.1. Gas-Phase Reaction Mechanism
3.2. Thermochemical Properties of the Intermediates
3.3. Effect of Solvent and Temperature
3.4. Comparison of the Relative Energies from G3MP2B3 and B3LYP/6-31G(d)
3.5. Thermochemistry of the Studied Reaction at Different Conditions
4. Conclusions
- (a)
- “Phosgenations first”: first amine groups turn into carbamic chloride groups and then the isocyanate groups form;
- (b)
- “Stepwise phosgenations”: amine group presented in ortho, or para, position converts as a carbamic chloride and then it loses HCl and forms isocyanate. The same step repeats for the other position and forms another isocyanate group.
- The addition of the first COCl2 is the rate limiting step for the reaction, regardless of which mechanism is taking place;
- The activation barriers for the gas-phase reaction are relatively high and the ODCB has reduced the activation barriers for all the reaction pathways;
- For transition states and intermediates, the gas phase and ODCB phase relative energies show strong linear correlation, and the presence of ODCB medium causes drastic shrinking in energy level of the transition state as one compares them with the gas-phase values. For the intermediates, the relative energies lowered by a factor of 1.18;
- Comparing the energy profile of the “phosgenations first” and “stepwise phosgenations”, the former has two exothermic consecutive elementary steps and then two consecutive endothermic ones, while the latter has consecutive exothermic and endothermic steps. Therefore, the “phosgenation first” seems to be the dominant channel due to both thermodynamic and kinetic points of view;
- Standard enthalpy of formation value is recommended for 2,4-TDA (59.3 kJ/mol) and 2,4-TDI (−94.1 kJ/mol), as well as for the gas-phase intermediates (IM);
- Relative energies obtained by G3MP2B3 and B3LYP/6-31G(d) has linear relation in both the gas phase and ODCB for transition states and intermediates. Therefore, B3LYP/6-31G(d) computation can be an excellent compromise between accuracy and computation time for phosgenation reactions.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Golling, F.E.; Pires, R.; Hecking, A.; Weikard, J.; Richter, F.; Danielmeier, K.; Dijkstra, D. Polyurethanes for Coatings and Adhesives—Chemistry and Applications. Polym. Int. 2019, 68, 848–855. [Google Scholar] [CrossRef]
- Lysien, K.; Stolarczyk, A.; Jarosz, T. Solid Propellant Formulations: A Review of Recent Progress and Utilized Components. Materials 2021, 14, 6657. [Google Scholar] [CrossRef] [PubMed]
- Parod, R.J. Toluene Diisocyanate, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 4, ISBN 9780123864543. [Google Scholar]
- TDI Global Demand 2021|Statista. Available online: https://www.statista.com/statistics/750818/tdi-demand-worldwide/ (accessed on 26 April 2022).
- Lorenz, W.; Padeken, L.; Pennemann, B.; Steffens, F.; Weismantel, L. Process for the Preparation of Toluene-Diisocyanate. U.S. Patent 8063241B2, 22 November 2011. [Google Scholar]
- Bähr, A.; Moon, G.H.; Diedenhoven, J.; Kiecherer, J.; Barth, E.; Tüysüz, H. Reactor Design and Kinetic Study on Adsorption/Desorption of CO and Cl2 for Industrial Phosgene Synthesis. Chemie Ing. Tech. 2018, 90, 1513–1519. [Google Scholar] [CrossRef]
- Bähr, A.; Diedenhoven, J.; Tüysüz, H. Cl2 Adsorption and Desorption over Ordered Mesoporous Carbon Materials as an Indicator for Catalytic Phosgene Formation. Chemie Ing. Tech. 2020, 92, 1508–1513. [Google Scholar] [CrossRef]
- Sohn, M.; Stroefer, E.; Nevejans, F.; Penzel, U.; Pallasch, H.-J.; Leuthold, R.; Brodhagen, A.; Woelfert, A.; Mackenroth, W.; Maurer, M. Method for the Continuous Production of Isocyanates. U.S. Patent 20060252960A1, 14 December 2010. [Google Scholar]
- Baldyga, J.; Molga, E.; Szarlik, S.; Wójcik, W.; Machniewski, P.; Rudniak, L.; Piechota, S.; Slawatycki, A.; Chrupala, W.; Lachmajer, J.; et al. A Method of Producing Toluene Diisocyanate (TDI) in the Process of the Toluene Diamine (TDA) Phosgenation Reaction in the Gaseous Phase. EP Patent 2463272A1, 28 October 2015. [Google Scholar]
- Pohl, F.D.; Serra, R.; Ehlers, M.; Bolton, J.S.; Solak, G.B.; Bourgeois, K.J.; Mccullough, G.L.; Hicks, A.R.; Hillman, R.G.; Sager, J.E.; et al. Process for the Continuous Preparation of Isocyanates. EP Patent 1873142A1, 4 November 2009. [Google Scholar]
- Suzuki, S.; Kurata, M.; Akiyoshi, A.; Aoshima, S.; Dan, H.; Matsuoka, N. Method of Manufacturing Toluene Diisocyanate. U.S. Patent 3484472A, 16 December 1969. [Google Scholar]
- Collas, G.; Gros, G.; Sagi, F. Separation of Toluene Diisocyanate from the Residues of the Production Thereof. U.S. Patent 4918220A, 17 April 1990. [Google Scholar]
- Iwanaga, K.; Seki, K.; Hibi, T.; Issoh, K.; Suzuta, T.; Nakada, M.; Mori, Y.; Abe, T. The Development of Improved Hydrogen Chloride Oxidation Process. Sumimoto Kagaku 2004, I, 1–11. [Google Scholar]
- Sanders, J.; Brummer, H.; Laue, J.; Sojka, B.; Eichmann, M.; Haverkamp, V. Gas-Phase Phosgenation Process. U.S. Patent 8692016B2, 8 April 2014. [Google Scholar]
- Boros, R.Z.; Koós, T.; Wafaa, C.; Nehéz, K.; Farkas, L.; Viskolcz, B.; Szőri, M. A Theoretical Study on the Phosgenation of Methylene Diphenyl Diamine (MDA). Chem. Phys. Lett. 2018, 706, 568–576. [Google Scholar] [CrossRef]
- Voßnacker, P.; Wüst, A.; Keilhack, T.; Müller, C.; Steinhauer, S.; Beckers, H.; Yogendra, S.; Schiesser, Y.; Weber, R.; Reimann, M.; et al. Novel Synthetic Pathway for the Production of Phosgene. Sci. Adv. 2021, 7, 5186–5215. [Google Scholar] [CrossRef] [PubMed]
- Lizardo-Huerta, J.C.; Sirjean, B.; Verdier, L.; Fournet, R.; Glaude, P.A. Thermal Decomposition of Phosgene and Diphosgene. J. Phys. Chem. A 2018, 122, 249–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lenzi, V.; Driest, P.J.; Dijkstra, D.J.; Ramos, M.M.D.; Marques, L.S.A. GAFF-IC: Realistic Viscosities for Isocyanate Molecules with a GAFF-Based Force Field. Mol. Simul. 2018, 45, 207–214. [Google Scholar] [CrossRef]
- Lenzi, V.; Driest, P.J.; Dijkstra, D.J.; Ramos, M.M.D.; Marques, L.S.A. Investigation on the Intermolecular Interactions in Aliphatic Isocyanurate Liquids: Revealing the Importance of Dispersion. J. Mol. Liq. 2019, 280, 25–33. [Google Scholar] [CrossRef] [Green Version]
- Cheikh, W.; Rózsa, Z.B.; López, C.O.C.; Mizsey, P.; Viskolcz, B.; Szori, M.; Fejes, Z. Urethane Formation with an Excess of Isocyanate or Alcohol: Experimental and Ab Initio Study. Polymers 2019, 11, 1543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waleed, H.Q.; Csécsi, M.; Hadjadj, R.; Thangaraj, R.; Pecsmány, D.; Owen, M.; Szőri, M.; Fejes, Z.; Viskolcz, B.; Fiser, B. Computational Study of Catalytic Urethane Formation. Polymers 2021, 14, 8. [Google Scholar] [CrossRef]
- Baboul, A.G.; Curtiss, L.A.; Redfern, P.C.; Raghavachari, K. Gaussian-3 Theory Using Density Functional Geometries and Zero-Point Energies. J. Chem. Phys. 1999, 110, 7650–7657. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, Revision E.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Peng, C.; Ayala, P.Y.; Schlegel, H.B.; Frisch, M.J. Using Redundant Internal Coordinates to Optimize Equilibrium Geometries and Transition States. J. Comput. Chem. 1996, 17, 49–56. [Google Scholar] [CrossRef]
- Dennington, R.; Keith, T.A.; Millam, J.M. GaussView, Version 6; Semichem Inc.: Shawnee Mission, KS, USA, 2016. [Google Scholar]
- Gonzalez, C.; Schlegel, H.B. An Improved Algorithm for Reaction Path Following. J. Chem. Phys. 1989, 90, 2154–2161. [Google Scholar] [CrossRef]
- NIST National Institute of Standards and Technology, NIST Chemistry Webbook, SRD 69, Group Additivity Based Estimates. Available online: https://webbook.nist.gov/chemistry/grp-add/ (accessed on 4 May 2022).
- NIST Computational Chemistry Comparison and Benchmark Database, NIST Standard Reference Database 101. Available online: https://cccbdb.nist.gov/ (accessed on 4 May 2022).
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on the Generalized Born Approximation with Asymmetric Descreening. J. Chem. Theory Comput. 2009, 5, 2447–2464. [Google Scholar] [CrossRef] [PubMed]
- Boros, R.Z. A Study of Elementary Reactions of Isocyanate Production. Ph.D. Thesis, University of Miskolc, Miskolc, Hungary, 2019. [Google Scholar]
- Uchimaru, T.; Yamane, S.; Mizukado, J.; Tsuzuki, S. Thermal Stabilities and Conformational Behaviors of Isocyanurates and Cyclotrimerization Energies of Isocyanates: A Computational Study. RSC Adv. 2020, 10, 15955–15965. [Google Scholar] [CrossRef] [PubMed] [Green Version]









| ΔE0,G3MP2B3 | ΔH°G3MP2B3 | ΔG°G3MP2B3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Solvent | - | ODCB | - | ODCB | - | ODCB | - | ODCB | - | ODCB |
| T in K | 0 | 298.15 | 423.15 | 298.15 | 423.15 | |||||
| TDA + 2COCl2 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| RC1 | 0.0 | −4.5 | 0.0 | −4.7 | 0.0 | −3.8 | 0.0 | 48.1 | 0.0 | 70.0 |
| RC2 | 0.0 | −11.5 | 0.0 | −11.1 | 0.0 | −10.0 | 0.0 | 38.4 | 0.0 | 58.9 |
| Tsa1 | 44.2 | −0.2 | 43.3 | −1.8 | 43.4 | −2.0 | 92.7 | 52.0 | 113.4 | 74.8 |
| IMa1 + HCl | −69.2 | −82.5 | −65.5 | −78.8 | −64.5 | −77.8 | −61.2 | −73.0 | −59.6 | −70.8 |
| Tsa2 + HCl | 43.6 | 6.4 | 48.1 | 10.2 | 48.9 | 10.8 | 48.4 | 15.9 | 48.4 | 18.0 |
| IMa2 + 2HCl | −51.3 | −59.1 | −42.8 | −50.6 | −41.4 | −49.3 | −83.5 | −89.1 | −100.8 | −105.5 |
| Tsa3 + 2HCl | 5.9 | −46.6 | 12.8 | −39.6 | 14.0 | −38.4 | 24.6 | −24.6 | 29.3 | −18.6 |
| IMa3 + 3HCl | −116.9 | −137.3 | −105.2 | −125.6 | −103.0 | −123.4 | −140.1 | −156.4 | −155.2 | −169.8 |
| Tsa4 + 3HCl | 4.7 | −42.5 | 17.3 | −30.3 | 19.4 | −28.4 | −21.1 | −62.5 | −37.7 | −77.6 |
| Tsa5 + HCl | −11.2 | −80.6 | −8.7 | −77.1 | −7.8 | −75.5 | 45.4 | −23.3 | 67.9 | −1.0 |
| IM4 + 2HCl | −137.5 | −161.9 | −130.3 | −154.5 | −128.4 | −152.6 | −120.3 | −143.1 | −116.5 | −138.7 |
| Tsa6 + 2HCl | −9.3 | −62.9 | −1.7 | −55.5 | 0.0 | −53.9 | 5.5 | −43.4 | 8.2 | −38.7 |
| TSb1 | 39.4 | −3.7 | 37.9 | −5.1 | 37.9 | −5.1 | 89.3 | 47.3 | 110.8 | 69.2 |
| Imb1 + HCl | −71.1 | −83.2 | −67.8 | −80.0 | −66.8 | −79.0 | −62.6 | −72.6 | −60.6 | −69.7 |
| TSb2 + HCl | 44.6 | 4.9 | 48.8 | 8.6 | 49.7 | 9.2 | 50.6 | 14.8 | 51.1 | 17.2 |
| Imb2 + 2HCl | −51.8 | −59.6 | −43.9 | −51.7 | −42.6 | −50.4 | −82.6 | −88.7 | −99.0 | −104.5 |
| TSb3 + 2HCl | 6.0 | −42.0 | 12.9 | −35.9 | 14.2 | −34.8 | 23.2 | −17.8 | 27.2 | −10.4 |
| Imb3 + 3HCl | −115.6 | −137.2 | −104.0 | −125.6 | −101.8 | −123.3 | −137.9 | −155.8 | −152.6 | −168.9 |
| TSb4 + 3HCl | 2.7 | −42.0 | 15.2 | −30.2 | 17.2 | −28.4 | −23.3 | −61.3 | −39.8 | −74.7 |
| TSb5 + HCl | −12.4 | −76.9 | −10.2 | −74.0 | −9.2 | −72.4 | 44.8 | −15.7 | 67.7 | 8.6 |
| TSb6 + 2HCl | −12.6 | −66.2 | −4.8 | −58.6 | −3.0 | −57.0 | 2.1 | −47.6 | 4.6 | −42.2 |
| TDI + 4HCl | −97.7 | −113.9 | −81.4 | −97.4 | −78.8 | −94.9 | −160.0 | −173.2 | −193.5 | −205.5 |
| Species | Δf,298.15KH0(g) (kJ/mol) (GA) | Δf,298.15KH0(g) (kJ/mol) (AS) | S0(g) (J/molK) | Cv(g) (J/molK) |
|---|---|---|---|---|
| TDA | 57.7 | 59.3 | 372.5 | 145.2 |
| IMa1 | n.a. | −135.5 | 454.3 | 183.2 |
| IMa2 | n.a. | −19.5 | 419.5 | 160.7 |
| IMa3 | n.a. | −211.2 | 496.6 | 197.3 |
| IM4 | n.a. | −329.7 | 531.7 | 220.5 |
| Imb1 | n.a. | −137.8 | 451.3 | 182.6 |
| Imb2 | n.a. | −20.6 | 412.8 | 159.7 |
| Imb3 | n.a. | −210.0 | 493.2 | 197.1 |
| TDI | −97.1 | −94.1 | 457.5 | 174.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thangaraj, R.; Horváth, T.; Boros, R.Z.; Viskolcz, B.; Szőri, M. A Theoretical Study on the Phosgenation of 2,4-Toluenediamine (2,4-TDA). Polymers 2022, 14, 2254. https://doi.org/10.3390/polym14112254
Thangaraj R, Horváth T, Boros RZ, Viskolcz B, Szőri M. A Theoretical Study on the Phosgenation of 2,4-Toluenediamine (2,4-TDA). Polymers. 2022; 14(11):2254. https://doi.org/10.3390/polym14112254
Chicago/Turabian StyleThangaraj, Ravikumar, Tamás Horváth, R. Zsanett Boros, Béla Viskolcz, and Milán Szőri. 2022. "A Theoretical Study on the Phosgenation of 2,4-Toluenediamine (2,4-TDA)" Polymers 14, no. 11: 2254. https://doi.org/10.3390/polym14112254