Assessment of the Efficiency of Chemical and Thermochemical Depolymerization Methods for Lignin Valorization: Principal Component Analysis (PCA) Approach
Abstract
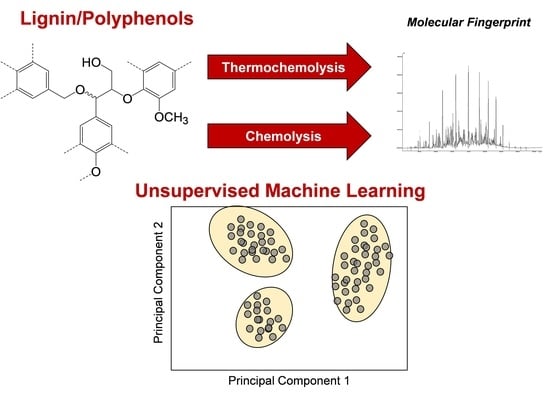
:1. Introduction
2. Materials and Methods
2.1. Peatland Samples
2.2. Sampling Strategy
2.3. Molecular Analyses
2.4. Principal Component Analysis (PCA)
3. CuO–NaOH Oxidation
4. Thermally Assisted Hydrolysis and Methylation (THM)
5. Results and Discussion
5.1. PCA CuO–NaOH
5.2. PCA TMAH
5.3. PCA for the Total Molecular Dataset (TMAH and CuO–NaOH)
5.4. Elemental Proxies and Factors Comparisons
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tarabanko, V.E.; Tarabanko, N. Catalytic oxidation of lignins into the aromatic aldehydes: General process trends and development prospects. Int. J. Mol. Sci. 2017, 18, 2421. [Google Scholar] [CrossRef] [Green Version]
- Tarabanko, V.E.; Kaygorodov, K.L.; Skiba, E.A.; Tarabanko, N.; Chelbina, Y.V.; Baybakova, O.V.; Kuznetsov, B.N.; Djakovitch, L. Processing pine wood into vanillin and glucose by sequential catalytic oxidation and enzymatic hydrolysis. J. Wood Chem. Technol. 2017, 37, 43–51. [Google Scholar] [CrossRef] [Green Version]
- Kazachenko, A.S.; Miroshnikova, A.V.; Tarabanko, V.E.; Skripnikov, A.M.; Malyar, Y.N.; Borovkova, V.S.; Sychev, V.V.; Taran, O.P. Thermal conversion of flax shives in sub-and supercritical ethanol in the presence of Ru/C catalyst. Catalysts 2021, 11, 970. [Google Scholar] [CrossRef]
- Shuai, L.; Amiri, M.T.; Questell-Santiago, Y.M.; Héroguel, F.; Li, Y.; Kim, H.; Meilan, R.; Chapple, C.; Ralph, J.; Luterbacher, J.S. Formaldehyde stabilization facilitates lignin monomer production during biomass depolymerization. Science 2016, 354, 329–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Z.; Bottari, G.; Afanasenko, A.; Stuart, M.C.A.; Deuss, P.J.; Fridrich, B.; Barta, K. Complete lignocellulose conversion with integrated catalyst recycling yielding valuable aromatics and fuels. Nat. Catal. 2018, 1, 82–92. [Google Scholar] [CrossRef]
- Ye, K.; Liu, Y.; Wu, S.; Zhuang, J. A review for lignin valorization: Challenges and perspectives in catalytic hydrogenolysis. Ind. Crops Prod. 2021, 172, 114008. [Google Scholar] [CrossRef]
- Britt, K.W. Handbook of Pulp and Paper Technology. In Handbook of Pulp and Paper Technology; Nab Nostrand Company: New York, NY, USA, 1970. [Google Scholar]
- Adler, E. Lignin chemistry—past, present and future. Wood Sci. Technol. 1977, 11, 169–218. [Google Scholar] [CrossRef]
- Younes, K.; Grasset, L. Comparison of thermochemolysis and classical chemical degradation and extraction methods for the analysis of carbohydrates, lignin and lipids in a peat bog. J. Anal. Appl. Pyrolysis 2018, 134, 61–72. [Google Scholar] [CrossRef]
- Zakzeski, J.; Bruijnincx, P.C.A.; Jongerius, A.L.; Weckhuysen, B.M. The catalytic valorization of lignin for the production of renewable chemicals. Chem. Rev. 2010, 110, 3552–3599. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, K.; Li, H.; Xiao, L.-P.; Song, G. Selective hydrogenolysis of catechyl lignin into propenylcatechol over an atomically dispersed ruthenium catalyst. Nat. Commun. 2021, 12, 1–9. [Google Scholar] [CrossRef]
- Cheng, C.; Truong, J.; Barrett, J.A.; Shen, D.; Abu-Omar, M.M.; Ford, P.C. Hydrogenolysis of organosolv lignin in ethanol/isopropanol media without added transition-metal catalyst. ACS Sustain. Chem. Eng. 2019, 8, 1023–1030. [Google Scholar] [CrossRef]
- Chowdari, R.K.; Agarwal, S.; Heeres, H.J. Hydrotreatment of kraft lignin to alkylphenolics and aromatics using Ni, Mo, and w phosphides supported on activated carbon. ACS Sustain. Chem. Eng. 2018, 7, 2044–2055. [Google Scholar] [CrossRef]
- Paananen, H.; Eronen, E.; Mäkinen, M.; Jänis, J.; Suvanto, M.; Pakkanen, T.T. Base-catalyzed oxidative depolymerization of softwood kraft lignin. Ind. Crops Prod. 2020, 152, 112473. [Google Scholar] [CrossRef]
- Fang, Q.; Jiang, Z.; Guo, K.; Liu, X.; Li, Z.; Li, G.; Hu, C. Low temperature catalytic conversion of oligomers derived from lignin in pubescens on Pd/NbOPO4. Appl. Catal. B. Environ. 2020, 263, 118325. [Google Scholar] [CrossRef]
- Kong, L.; Zhang, L.; Gu, J.; Gou, L.; Xie, L.; Wang, Y.; Dai, L. Catalytic hydrotreatment of kraft lignin into aromatic alcohols over nickel-rhenium supported on niobium oxide catalyst. Bioresour. Technol. 2020, 299, 122582. [Google Scholar] [CrossRef]
- Wu, Z.; Zhao, X.; Zhang, J.; Li, X.; Zhang, Y.; Wang, F. Ethanol/1,4-dioxane/formic acid as synergistic solvents for the conversion of lignin into high-value added phenolic monomers. Bioresour. Technol. 2019, 278, 187–194. [Google Scholar] [CrossRef]
- Ma, R.; Guo, M.; Zhang, X. Recent advances in oxidative valorization of lignin. Catal. Today 2018, 302, 50–60. [Google Scholar] [CrossRef]
- Ertel, J.R.; Hedges, J.I. Sources of sedimentary humic substances: Vascular plant debris. Geochim. Cosmochim. Acta 1985, 49, 2097–2107. [Google Scholar] [CrossRef]
- Hedges, J.I.; Blanchette, R.A.; Weliky, K.; Devol, A.H. Effects of fungal degradation on the CuO oxidation products of lignin: A controlled laboratory study. Geochim. Cosmochim. Acta 1988, 52, 2717–2726. [Google Scholar] [CrossRef]
- Shadkami, F.; Helleur, R. Recent applications in analytical thermochemolysis. J. Anal. Appl. Pyrolysis 2010, 89, 2–16. [Google Scholar] [CrossRef]
- Younes, K.; Grasset, L. Analysis of molecular proxies of a peat core by thermally assisted hydrolysis and methylation-gas chromatography combined with multivariate analysis. J. Anal. Appl. Pyrolysis 2017, 124, 726–732. [Google Scholar] [CrossRef]
- Chapman, S.; Buttler, A.; Francez, A.-J.; Laggoun-Défarge, F.; Vasander, H.; Schloter, M.; Combe, J.; Grosvernier, P.; Harms, H.; Epron, D. Exploitation of northern peatlands and biodiversity maintenance: A conflict between economy and ecology. Front. Ecol. Environ. 2003, 1, 525–532. [Google Scholar] [CrossRef]
- Delarue, F.; Laggoun-Défarge, F.; Buttler, A.; Gogo, S.; Jassey, V.E.; Disnar, J.-R. Effects of short-term ecosystem experimental warming on water-extractable organic matter in an ombrotrophic sphagnum peatland (Le Forbonnet, France). Org. Geochem. 2011, 42, 1016–1024. [Google Scholar] [CrossRef] [Green Version]
- Biester, H.; Knorr, K.-H.; Schellekens, J.; Basler, A.; Hermanns, Y.-M. Comparison of different methods to determine the degree of peat decomposition in peat bogs. Biogeosciences 2014, 11, 2691–2707. [Google Scholar] [CrossRef] [Green Version]
- Younes, K.; Laduranty, J.; Descostes, M.; Grasset, L. Molecular biomarkers study of an ombrotrophic peatland impacted by an anthropogenic clay deposit. Org. Geochem. 2017, 105, 20–32. [Google Scholar] [CrossRef]
- Schellekens, J.; Buurman, P.; Kuyper, T.W.; Abbott, G.D.; Pontevedra-Pombal, X.; Martínez-Cortizas, A. Influence of source vegetation and redox conditions on lignin-based decomposition proxies in graminoid-dominated ombrotrophic peat (Penido Vello, NW Spain). Geoderma 2015, 237, 270–282. [Google Scholar] [CrossRef] [Green Version]
- Clymo, R.S.; Turunen, J.; Tolonen, K. Carbon accumulation in Peatland. Oikos 1998, 81, 368–388. [Google Scholar] [CrossRef] [Green Version]
- Younes, K.; Grasset, L. The Application of DFRC method for the analysis of carbohydrates in a peat bog: Validation and comparison with conventional chemical and thermochemical degradation techniques. Chem. Geol. 2020, 545, 119644. [Google Scholar] [CrossRef]
- Bordelet, G. Etude l’adsorption et de La Désorption de 226RA (II) et 238U (VI) Dans La Matière Organique de La Tourbe, En Contexte Minier. PhD Thesis, University of Évry Val d’Essonne, Paris, France, 2014. [Google Scholar]
- Wang, Y.; Frutschi, M.; Suvorova, E.; Phrommavanh, V.; Descostes, M.; Osman, A.A.; Geipel, G.; Bernier-Latmani, R. Mobile uranium (IV)-bearing colloids in a mining-impacted wetland. Nat. Commun. 2013, 4, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Bagnoud, A.; Suvorova, E.; McGivney, E.; Chesaux, L.; Phrommavanh, V.; Descostes, M.; Bernier-Latmani, R. Geochemical control on uranium (IV) mobility in a mining-impacted wetland. Environ. Sci. Technol. 2014, 48, 10062–10070. [Google Scholar] [CrossRef] [Green Version]
- Boekhout, F.; Gérard, M.; Kanzari, A.; Michel, A.; Déjeant, A.; Galoisy, L.; Calas, G.; Descostes, M. Uranium migration and retention during weathering of a granitic waste rock pile. Appl. Geochem. 2015, 58, 123–135. [Google Scholar] [CrossRef]
- Jolliffe, I.T. Principal component analysis. In Springer Series in Statistics; Springer Nature: Cham, Switzerland, 2005; p. 29. [Google Scholar]
- Joliffe, I.T.; Morgan, B.J.T. Principal component analysis and exploratory factor analysis. Stat. Methods Med. Res. 1992, 1, 69–95. [Google Scholar] [CrossRef] [PubMed]
- Younes, K.; Mouhtady, O.; Chaouk, H.; Obeid, E.; Roufayel, R.; Moghrabi, A.; Murshid, N. The application of principal component analysis (PCA) for the optimization of the conditions of fabrication of electrospun nanofibrous membrane for desalination and ion removal. Membranes 2021, 11, 979. [Google Scholar] [CrossRef]
- Korichi, W.; Ibrahimi, M.; Loqman, S.; Ouhdouch, Y.; Younes, K.; Lemée, L. Assessment of actinobacteria use in the elimination of multidrug-resistant bacteria of ibn tofail hospital wastewater (Marrakesh, Morocco): A chemometric data analysis approach. Environ. Sci. Pollut Res. 2021, 28, 26840–26848. [Google Scholar] [CrossRef] [PubMed]
- Bahri, H.; Rasse, D.P.; Rumpel, C.; Dignac, M.-F.; Bardoux, G.; Mariotti, A. Lignin degradation during a laboratory incubation followed by 13C Isotope analysis. Soil Biol. Biochem. 2008, 40, 1916–1922. [Google Scholar] [CrossRef]
- Kögel-Knabner, I. Organische Stoffgruppen in Waldhumusformen Und Ihr Verhalten Während Der Streuzersetzung Und Humifizierung. PhD Thesis, Lehrstuhl für Bodenkunde und Bodengeographie, Universität Bayreuth, Bayern, Germany, 1987. [Google Scholar]
- Thevenot, M.; Dignac, M.-F.; Rumpel, C. Fate of lignins in soils: A Review. Soil Biol. Biochem. 2010, 42, 1200–1211. [Google Scholar] [CrossRef]
- Opsahl, S.; Benner, R. Characterization of carbohydrates during early diagenesis of five vascular plant tissues. Org. Geochem. 1999, 30, 83–94. [Google Scholar] [CrossRef]
- Amelung, W.; Flach, K.-W.; Zech, W. Lignin in particle-size fractions of native grassland soils as influenced by climate. Soil Sci. Soc. Am. J. 1999, 63, 1222–1228. [Google Scholar] [CrossRef] [Green Version]
- Rumpel, C.; Eusterhues, K.; Kögel-Knabner, I. Location and chemical composition of stabilized organic carbon in topsoil and subsoil horizons of two acid forest soils. Soil Biol. Biochem. 2004, 36, 177–190. [Google Scholar] [CrossRef]
- Otto, A.; Simpson, M.J. Evaluation of CuO oxidation parameters for determining the source and stage of lignin degradation in Soil. Biogeochemistry 2006, 80, 121–142. [Google Scholar] [CrossRef]
- Downing, D.T. Analysis of aqueous solutions of organic acids by pyrolysis of their tetramethylammonium salts in the gas chromatograph. Anal. Chem. 1967, 39, 218–221. [Google Scholar] [CrossRef]
- Challinor, J.M. The development and applications of thermally assisted hydrolysis and methylation reactions. J. Anal. Appl. Pyrolysis 2001, 61, 3–34. [Google Scholar] [CrossRef]
- Steinberg, S.M.; Nemr, E.L.; Rudin, M. Characterization of the lignin signature in lake mead, NV, sediment: Comparison of on-line flash chemopyrolysis (600 C) and off-line chemolysis (250 C). Environ. Geochem. Health 2009, 31, 339. [Google Scholar] [CrossRef]
- Kuroda, K.; Nakagawa-izumi, A. Tetramethylammonium hydroxide (TMAH) thermochemolysis of lignin: Behavior of 4- O -etherified cinnamyl alcohols and aldehydes. J. Agric. Food Chem. 2005, 53, 8859–8865. [Google Scholar] [CrossRef]
- Kuroda, K.; Nakagawa-izumi, A. Tetramethylammonium hydroxide thermochemolysis of guaiacyl–syringyl and guaiacyl dehydrogenation polymers. Org. Geochem. 2005, 36, 53–61. [Google Scholar] [CrossRef]
- Kuroda, K.-I.; Nakagawa-izumi, A. Tetramethylammonium hydroxide (TMAH) thermochemolysis of lignin: Improvement of the distribution profile of products derived from β-aryl ether subunits. J. Anal. Appl. Pyrolysis 2006, 75, 104–111. [Google Scholar] [CrossRef]
- Kuroda, K.; Nakagawa-izumi, A.; Ashitani, T.; Fujita, K. Tetramethylammonium hydroxide (TMAH) thermochemolysis of 2-arylcoumaran lignin model compounds. J. Anal. Appl. Pyrolysis 2009, 86, 185–191. [Google Scholar] [CrossRef]
- Nakagawa-izumi, A.; Kuroda, K.; Ozawa, T. Thermochemolytic behavior of β–β lignin structures in the presence of tetramethylammonium hydroxide (TMAH). Org. Geochem. 2004, 35, 763–774. [Google Scholar] [CrossRef]
- Wysocki, L.A.; Filley, T.R.; Bianchi, T.S. Comparison of two methods for the analysis of lignin in marine sediments: CuO oxidation versus tetramethylammonium hydroxide (TMAH) thermochemolysis. Org. Geochem. 2008, 39, 1454–1461. [Google Scholar] [CrossRef]
- Klotzbücher, T.; Filley, T.R.; Kaiser, K.; Kalbitz, K. A study of lignin degradation in leaf and needle litter using 13C-labelled tetramethylammonium hydroxide (TMAH) thermochemolysis: Comparison with CuO oxidation and van soest methods. Org. Geochem. 2011, 42, 1271–1278. [Google Scholar] [CrossRef]
- Bourdon, S.; Laggoun-Défarge, F.; Disnar, J.-R.; Maman, O.; Guillet, B.; Derenne, S.; Largeau, C. Organic matter sources and early diagenetic degradation in a tropical peaty marsh (Tritrivakely, Madagascar). Implications for environmental reconstruction during the Sub-Atlantic. Org. Geochem. 2000, 31, 421–438. [Google Scholar] [CrossRef] [Green Version]
- Anderson, H.; Hepburn, A. Variation of Humic Substances within Peat Profile; Peat and Water Academic Press: New York, NY, USA, 1986; pp. 177–194. [Google Scholar]
- Zaccone, C.; Miano, T.M.; Shotyk, W. Qualitative comparison between raw peat and related humic acids in an ombrotrophic bog profile. Org. Geochem. 2007, 38, 151–160. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Younes, K.; Moghrabi, A.; Moghnie, S.; Mouhtady, O.; Murshid, N.; Grasset, L. Assessment of the Efficiency of Chemical and Thermochemical Depolymerization Methods for Lignin Valorization: Principal Component Analysis (PCA) Approach. Polymers 2022, 14, 194. https://doi.org/10.3390/polym14010194
Younes K, Moghrabi A, Moghnie S, Mouhtady O, Murshid N, Grasset L. Assessment of the Efficiency of Chemical and Thermochemical Depolymerization Methods for Lignin Valorization: Principal Component Analysis (PCA) Approach. Polymers. 2022; 14(1):194. https://doi.org/10.3390/polym14010194
Chicago/Turabian StyleYounes, Khaled, Ahmad Moghrabi, Sara Moghnie, Omar Mouhtady, Nimer Murshid, and Laurent Grasset. 2022. "Assessment of the Efficiency of Chemical and Thermochemical Depolymerization Methods for Lignin Valorization: Principal Component Analysis (PCA) Approach" Polymers 14, no. 1: 194. https://doi.org/10.3390/polym14010194