A Novel Generation of Polysulfone/Crown Ether-Functionalized Reduced Graphene Oxide Membranes with Potential Applications in Hemodialysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Functionalization of rGO-NH2 with CE
2.2. Preparation of the PSF/rGO-NH2-CE Composite Membranes
2.3. Metal Ions Retention Efficiency
2.4. Physico-Chemical Characterization
3. Results
3.1. Characterization of Functionalized rGO-NH2
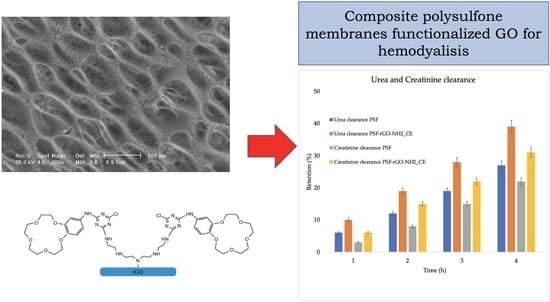
3.2. Characterization of PSF/rGO-NH2-CE Composite Membranes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. Exp. Suppl. 2012, 101, 133–164. [Google Scholar]
- Alissa, E.M.; Ferns, G.A. Heavy metal poisoning and cardiovascular disease. J. Toxicol. 2011, 2011, 870125. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Gautam, N.; Mishra, A.; Gupta, R. Heavy metals and living systems: An overview. Indian J. Pharmacol. 2011, 43, 246–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mondal, S.; Kumar Majumder, S. Fabrication of the polysulfone-based composite ultrafiltration membranes for the adsorptive removal of heavy metal ions from their contaminated aqueous solutions. Chem. Eng. J. 2020, 401, 126036. [Google Scholar] [CrossRef]
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium, Cadmium, and Arsenic. Front. Pharmacol. 2021, 12, 643972. [Google Scholar] [CrossRef] [PubMed]
- Ronco, C.; Clark, W.R. Haemodialysis membranes. Nat. Rev. Nephrol. 2018, 14, 394–410. [Google Scholar] [CrossRef]
- To, N.; Sanada, I.; Ito, H.; Prihandana, G.S.; Morita, S.; Kanno, Y.; Miki, N. Water-permeable dialysis membranes for multi-layered microdialysis system. Front. Bioeng. Biotechnol. 2015, 3, 70. [Google Scholar] [CrossRef]
- Wenten, I.G.; Aryanti, P.T.P.; Khoiruddin, K.; Hakim, A.N.; Himma, N.F. Advances in Polysulfone-Based Membranes for Hemodialysis. J. Membr. Sci. Res. 2016, 2, 78–89. [Google Scholar]
- Yamashita, A.C.; Sakurai, K. Updates in Hemodialysis; Suzuki, H., Ed.; IntechOpen: London, UK, 2015. [Google Scholar]
- Eduok, U.; Abdelrasoul, A.; Shoker, A.; Doan, H. Recent Developments, Current Challenges and Future Perspectives on Cellulosic Hemodialysis Membranes for Highly Efficient Clearance of Uremic Toxins. Mater. Today Commun. 2021, 27, 102183. [Google Scholar] [CrossRef]
- Clark, W.R.; Hamburger, R.J.; Lysaght, M.J. Effect of membrane composition and structure on solute removal and biocompatibility in hemodialysis. Kidney Int. 1999, 56, 2005–2015. [Google Scholar] [CrossRef] [Green Version]
- Poppelaars, F.; Faria, B.; Gaya da Costa, M.; Franssen, C.F.M.; van Son, W.J.; Berger, S.P.; Daha, M.R.; Seelen, M.A. The Complement System in Dialysis: A Forgotten Story? Front. Immunol. 2018, 9, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouré, T.; Vanholder, R. Which dialyser membrane to choose? Nephrol. Dial. Transplant. 2004, 19, 293–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jørstad, S.; Smeby, L.C.; Balstad, T.; Widerøe, T.E. Generation and removal of anaphylatoxins during hemofiltration with five different membranes. Blood Purif. 1988, 6, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Zhong, D.; Wang, Z.; Zhou, J.; Wang, Y. Additive-free preparation of hemodialysis membranes from block copolymers of polysulfone and polyethylene glycol. J. Membr. Sci. 2021, 618, 118690. [Google Scholar] [CrossRef]
- Wang, C.; Lin, B.; Qiu, Y. Enhanced hydrophilicity and anticoagulation of polysulfone materials modified via dihydroxypropyl, sulfonic groups and chitosan. Colloids Surf. B 2022, 210, 112243. [Google Scholar] [CrossRef] [PubMed]
- Abidin, M.N.Z.; Goh, P.S.; Said, N.; Ismail, A.F.; Othman, M.H.D.; Abdullah, M.S.; Ng, B.C.; Hasbullah, H.; Sheikh Abdul Kadir, S.H.; Kamal, F.; et al. Polysulfone/amino-silanized poly (methyl methacrylate) dual layer hollow fiber membrane for uremic toxin separation. Sep. Purif. Technol. 2020, 236, 116216. [Google Scholar] [CrossRef]
- Qi, X.; Yang, N.; Luo, Y.; Jia, X.; Zhao, J.; Feng, X.; Chen, L.; Zhao, Y. Resveratrol as a plant type antioxidant modifier for polysulfone membranes to improve hemodialysis-induced oxidative stress. Mater. Sci. Eng. C 2021, 123, 111953. [Google Scholar] [CrossRef]
- Yang, N.; Jia, X.; Wang, D.; Wei, C.; He, Y.; Chen, L.; Zhao, Y. Silibinin as a natural antioxidant for modifying polysulfone membranes to suppress hemodialysis-induced oxidative stress. J. Membr. Sci. 2018, 574, 86–99. [Google Scholar] [CrossRef]
- Mahlicli, F.; Altinkaya, S. Immobilization of alpha lipoic acid onto polysulfone membranes to suppress hemodialysis induced oxidative stress. J. Membr. Sci. 2014, 449, 27. [Google Scholar] [CrossRef] [Green Version]
- Tu, M.-M.; Xu, J.-J.; Qiu, Y.-R. Surface hemocompatible modification of polysulfone membrane via covalently grafting acrylic acid and sulfonated hydroxypropyl chitosan. RSC Adv. 2019, 9, 6254–6266. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, G.P.S.; Isloor, A.M.; Inamuddin; Asiri, A.M.; Ismail, A.F.; Kumar, R.; Ahamed, M.I. Performance intensification of the polysulfone ultrafiltration membrane by blending with copolymer encompassing novel derivative of poly (styrene-co-maleic anhydride) for heavy metal removal from wastewater. Chem. Eng. J. 2018, 353, 425–435. [Google Scholar] [CrossRef]
- Benkhaya, S.; Lgaz, H.; Alrashdi, A.A.; M’Rabet, S.; El Bachiri, A.; Assouag, M.; Chung, I.-M.; El Harfi, A. Upgrading the performances of polysulfone/polyetherimide ultrafiltration composite membranes for dyes removal: Experimental and molecular dynamics studies. J. Mol. Liq. 2021, 331, 115743. [Google Scholar] [CrossRef]
- Kim, K.; Lee, K.; Cho, K.; Park, C. Surface modification of polysulfone ultrafiltration by oxygen plasma treatment. J. Membr. Sci. 2002, 199, 135–145. [Google Scholar] [CrossRef]
- Serbanescu, O.S.; Voicu, S.I.; Thakur, V.K. Polysulfone functionalized membranes: Properties and challenges. Mater. Today Chem. 2020, 17, 100302. [Google Scholar] [CrossRef]
- RefaatAlawady, A.; Ali Alshahrani, A.; Ali Aouak, T.; Mohamed Alandis, N. Polysulfone membranes with CNTs/Chitosan biopolymer nanocomposite as selective layer for remarkable heavy metal ions rejection capacity. Chem. Eng. J. 2020, 388, 124267. [Google Scholar] [CrossRef]
- Sherugar, P.; Naik, N.S.; Padaki, M.; Nayak, V.; Gangadharan, A.; Nadig, A.R.; Déon, S. Fabrication of zinc doped aluminium oxide/polysulfone mixed matrix membranes for enhanced antifouling property and heavy metal removal. Chemosphere 2021, 275, 130024. [Google Scholar] [CrossRef]
- Ionita, M.; Pandele, A.M.; Crica, L.; Pilan, L. Improving the thermal and mechanical properties of polysulfone by incorporation of graphene oxide. Compos. B Eng. 2014, 59, 133–139. [Google Scholar] [CrossRef]
- Mamah, S.C.; Goh, P.S.; Ismail, A.F.; Suzaimi, N.D.; Yogarathinam, L.T.; Raji, Y.O.; El-badawy, T.H. Recent development in modification of polysulfone membrane for water treatment application. J. Water Proc. Eng. 2021, 40, 101835. [Google Scholar] [CrossRef]
- Serbanescu, O.S.; Pandele, A.M.; Miculescu, F.; Voicu, S.I. Synthesis and Characterization of Cellulose Acetate Membranes with Self-Indicating Properties by Changing the Membrane Surface Color for Separation of Gd (III). Coatings 2020, 10, 468. [Google Scholar] [CrossRef]
- Serbanescu, O.; Pandele, A.; Oprea, M.; Semenescu, A.; Thakur, V.K.; Voicu, Ş.I. Crown Ether-Immobilized Cellulose Acetate Membranes for the Retention of Gd (III). Polymers 2021, 13, 3978. [Google Scholar] [CrossRef] [PubMed]
- Voicu, S.; Thakur, V.K. Graphene-based composite membranes for nanofiltration: Performances and future perspectives. Emerg. Mater. 2021, 1–13. [Google Scholar] [CrossRef]
- Muhulet, A.; Tuncel, C.; Miculescu, F.; Pandele, A.M.; Bobirica, C.; Orbeci, C.; Bobirica, L.; Palla-Papavlu, A.; Voicu, S.I. Synthesis and characterization of polysulfone–TiO2 decorated MWCNT composite membranes by sonochemical method. Appl. Phys. A 2020, 126, 233. [Google Scholar] [CrossRef]
- Pandele, A.M.; Iovu, H.; Orbeci, C.; Tuncel, C.; Miculescu, F.; Nicolescu, A.; Deleanu, C.; Voicu, S.I. Surface modified cellulose acetate membranes for the reactive retention of tetracycline. Sep. Purif. Technol. 2020, 249, 117145. [Google Scholar] [CrossRef]
- Voicu, S.I.; Thakur, V.K. Aminopropyltriethoxysilane as a linker for cellulose-based functional materials: New horizons and future challenges. Curr. Opin. Green Sustain. Chem. 2021, 30, 100480. [Google Scholar] [CrossRef]
- Pandele, A.M.; Constantinescu, A.; Radu, I.C.; Miculescu, F.; Ioan Voicu, S.; Ciocan, L.T. Synthesis and Characterization of PLA-Micro-structured Hydroxyapatite Composite Films. Materials 2020, 13, 274. [Google Scholar] [CrossRef] [Green Version]
- Voicu, S.; Dobrica, A.; Sava, S.; Ivan, A.; Naftanaila, L. Cationic surfactants-controlled geometry and dimensions of polymeric membrane pores. J. Optoelectron. Adv. Mater. 2012, 14, 923–928. [Google Scholar]
- Rana, A.K.; Gupta, V.K.; Saini, A.K.; Voicu, S.I.; Abdellattifaand, M.H.; Thakur, V.K. Water desalination using nanocelluloses/cellulose derivatives based membranes for sustainable future. Desalination 2021, 520, 115359. [Google Scholar] [CrossRef]
- Chiulan, I.; Heggset, E.B.; Voicu, Ş.I.; Chinga-Carrasco, G. Photopolymerization of Bio-Based Polymers in a Biomedical Engineering Perspective. Biomacromolecules 2021, 22, 1795–1814. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Cheng, H.-M. The Fabrication, Properties, and Uses of Graphene/Polymer Composites. Macromol. Chem. Phys. 2012, 213, 1060–1077. [Google Scholar] [CrossRef]
- Zhang, T.; Xue, Q.; Zhang, S.; Dong, M. Theoretical approaches to graphene and graphene-based materials. Nano Today 2012, 7, 180–200. [Google Scholar] [CrossRef]
- Pei, S.; Cheng, H.-M. The reduction of graphene oxide. Carbon 2012, 50, 3210–3228. [Google Scholar] [CrossRef]
- Banerjee, A.N. Graphene and its derivatives as biomedical materials: Future prospects and challenges. Interface Focus 2018, 8, 20170056. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Chakraborty, S.; Monikh, F.A.; Varsou, D.-D.; Chetwynd, A.J.; Afantitis, A.; Lynch, I.; Zhang, P. Surface Functionalization of Graphene-Based Materials: Biological Behavior, Toxicology, and Safe-By-Design Aspects. Adv. Biol. 2021, 5, 2100637. [Google Scholar] [CrossRef] [PubMed]
- Oprea, M.; Voicu, S.I. Cellulose Composites with Graphene for Tissue Engineering Applications. Materials 2020, 13, 5347. [Google Scholar] [CrossRef] [PubMed]
- Bandehali, S.; Moghadassi, A.; Parvizian, F.; Zhang, Y.; Hosseini, S.M.; Shen, J. New mixed matrix PEI nanofiltration membrane decorated by glycidyl-POSS functionalized graphene oxide nanoplates with enhanced separation and antifouling behaviour: Heavy metal ions removal. Sep. Purif. Technol. 2020, 242, 116745. [Google Scholar] [CrossRef]
- Petrescu, S.; Avramescu, S.; Musuc, A.M.; Neatu, F.; Florea, M.; Ionita, P. Crown-ether functionalized graphene oxide for metal ions sequestration. Mater. Res. Bull. 2020, 122, 110643. [Google Scholar] [CrossRef]
- Nisola, G.M.; Parohinog, K.J.; Cho, M.K.; Burnea, F.K.B.; Lee, J.Y.; Seo, J.G.; Lee, S.-P.; Chung, W.-J. Covalently decorated crown ethers on magnetic graphene oxides as bi-functional adsorbents with tailorable ion recognition properties for selective metal ion capture in water. Chem. Eng. J. 2020, 389, 123421. [Google Scholar] [CrossRef]
- Seetharaman, S.; Raghu, S.C.; Velan, M.; Ramya, K.; Mahabadi, K.A. Comparison of the performance of reduced graphene oxide and multiwalled carbon nanotubes based sulfonated polysulfone membranes for electrolysis application. Polym. Compos. 2015, 36, 475–481. [Google Scholar] [CrossRef]
- Davis, F.; Higson, S. Macrocycles: Construction, Chemistry and Nanotechnology Applications; Wiley: Hoboken, NJ, USA, 2011; pp. 34–76. [Google Scholar]
- Song, L.; Huo, J.; Wang, X.; Yang, F.; He, J.; Li, C. Phosphate adsorption by a Cu (II)-loaded polyethersulfone-type metal affinity membrane with the presence of coexistent ions. Chem. Eng. J. 2016, 284, 182–193. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Z.; Luo, F.; Peng, H.-Y.; Zhang, S.-G.; Xie, R.; Ju, X.-J.; Wang, W.; Faraj, Y.; Chu, L.-Y. A novel smart membrane with ion-recognizable nanogels as gates on interconnected pores for simple and rapid detection of trace lead (II) ions in water. J. Membr. Sci. 2019, 575, 28–37. [Google Scholar] [CrossRef]
- Jin, C.; Liu, G.; Wu, G.; Huo, S.; Liu, Z.; Kong, Z. Facile fabrication of crown ether functionalized lignin-based biosorbent for the selective removal of Pb (II). Ind. Crops Prod. 2020, 155, 112829. [Google Scholar] [CrossRef]
- Kazemzadeh, H.; Karimi-Sabet, J.; Towfighi Darian, J.; Adhami, A. Evaluation of polymer inclusion membrane efficiency in selective separation of lithium ion from aqueous solution. Sep. Purif. Technol. 2020, 251, 117298. [Google Scholar] [CrossRef]
- Liu, C.; Walter, D.; Neuhauser, D.; Baer, R. Molecular Recognition and Conductance in Crown Ethers. J. Am. Chem. Soc. 2003, 125, 13936–13937. [Google Scholar] [CrossRef]
- Korchowiec, B.; Trojan, S.; Joly, J.-P.; Korchowiec, J.; Beley, M.; Rogalska, E. The interaction of an amphiphile crown ether with divalent metal ions. An electrochemical, Langmuir film, and molecular modeling study. Thin Solid Films 2019, 683, 49–56. [Google Scholar] [CrossRef]
- Wan, W.; Zhao, Z.; Hu, H.; Gogotsi, Y.; Qiu, J. Highly controllable and green reduction of graphene oxide to flexible graphene film with high strength. Mater. Res. Bull. 2013, 48, 4797–4803. [Google Scholar] [CrossRef]
- Sharma, M.; Rani, S.; Pathak, D.K.; Bhatia, R.; Kumar, R.; Sameera, I. Temperature dependent Raman modes of reduced graphene oxide: Effect of anharmonicity, crystallite size and defects. Carbon 2021, 184, 437–444. [Google Scholar] [CrossRef]
- Pandele, A.M.; Ionita, M.; Lungu, A.; Vasile, E.; Zaharia, C.; Iovu, H. Porous chitosan/graphene oxide biocomposites for tissue engineering. Polym. Compos. 2017, 38, 363–370. [Google Scholar] [CrossRef]
- Eigler, S.; Dotzer, C.; Hirsch, A. Visualization of defect densities in reduced graphene oxide. Carbon 2012, 50, 3666–3673. [Google Scholar] [CrossRef]
- Olsen, G.; Ulstrup, J.; Chi, Q. Crown-Ether Derived Graphene Hybrid Composite for Membrane-Free Potentiometric Sensing of Alkali Metal Ions. ACS Appl. Mater. Interf. 2016, 8, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Pandele, A.M.; Andronescu, C.; Vasile, E.; Radu, I.C.; Stanescu, P.; Iovu, H. Non-covalent functionalization of GO for improved mechanical performances of pectin composite films. Compos. A Appl. Sci. Manuf. 2017, 103, 188–195. [Google Scholar] [CrossRef]
- Ayiania, M.; Smith, M.; Hensley, A.J.; Scudiero, L.; McEwen, J.-S.; Garcia-Perez, M. Deconvoluting the XPS spectra for nitrogen-doped chars: An analysis from first principles. Carbon 2020, 162, 528–544. [Google Scholar] [CrossRef]
- Velásquez-Rojas, M.M.; Contreras-Torres, F.F.; Meza-Laguna, V.; Álvarez-Zauco, E.; Farías, M.H.; Basiuk, V.A.; Basiuk, E.V. Solvent-free functionalization of graphene oxide powder and paper with aminobenzo-crown ethers and complexation with alkali metal cations. Mater. Chem. Phys. 2021, 260, 124127. [Google Scholar] [CrossRef]
- Mironova, M.; Makarov, I.; Golova, L.; Vinogradov, M.; Shandryuk, G.; Levin, I. Improvement in Carbonization Efficiency of Cellulosic Fibres Using Silylated Acetylene and Alkoxysilanes. Fibers 2019, 7, 84. [Google Scholar] [CrossRef] [Green Version]
- Ficai, D.; Ficai, A.; Voicu, G.; Vasile, B.; Guran, C.; Andronescu, E. Polysulfone based Membranes with Desired Pores Characteristics. Mater. Plast. 2010, 47, 24–27. [Google Scholar]
- Alkhouzaam, A.; Qiblawey, H. Novel polysulfone ultrafiltration membranes incorporating polydopamine functionalized graphene oxide with enhanced flux and fouling resistance. J. Membr. Sci. 2021, 620, 118900. [Google Scholar] [CrossRef]
- Sharma, N.; Sharma, V.; Jain, Y.; Kumari, M.; Gupta, R.; Sharma, S.K.; Sachdev, K. Synthesis and Characterization of Graphene Oxide (GO) and Reduced Graphene Oxide (rGO) for Gas Sensing Application. Macromol. Sympos. 2017, 376, 1700006. [Google Scholar] [CrossRef]
- Voicu, Ş.I.; Pandele, A.; Vasile, E.; Rughinis, R.; Crica, L.; Pilan, L.; Ionita, M. The impact of sonication time through polysulfone-graphene oxide composite films properties. Dig. J. Nanomat. Biostruct. 2013, 8, 1389–1394. [Google Scholar]
- Oprea, M.; Voicu, S.I. Recent Advances in Applications of Cellulose Derivatives-Based Composite Membranes with Hydroxyapatite. Materials 2020, 13, 2481. [Google Scholar] [CrossRef]
- Kabsch-Korbutowicz, M.; Winnicki, T. Application of modified polysulfone membranes to the treatment of water solutions containing humic substances and metal ions. Desalination 1996, 105, 41–49. [Google Scholar] [CrossRef]
- Molinari, R.; Gallo, S.; Argurio, P. Metal ions removal from wastewater or washing water from contaminated soil by ultrafiltration–complexation. Water Res. 2004, 38, 593–600. [Google Scholar] [CrossRef]












| Chemical Bond | Wavenumber (cm−1) |
|---|---|
| N–H (amide II) | 1651 |
| N–H (amine) | 1549 |
| C–N | 1440 |
| C–O (epoxy) | 1166 |
| C–O (ether) | 1087 |
| Atomic Percentage [%] | ||
|---|---|---|
| rGO-NH2 | rGO-NH2-CE | |
| C 1s | 82.97 | 78.78 |
| O 1s | 9.02 | 13.25 |
| N 1s | 8.01 | 7.97 |
| Sample | T10% [°C] | Tmax [°C] | R800 [%] | WL100 [%] |
|---|---|---|---|---|
| rGO-NH2 | 160.26 | 179.15 | 38.17 | 1.95 |
| rGO-NH2-CE | 300.44 | 344.48 | 46.33 | 2.75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pandele, A.M.; Oprea, M.; Dutu, A.A.; Miculescu, F.; Voicu, S.I. A Novel Generation of Polysulfone/Crown Ether-Functionalized Reduced Graphene Oxide Membranes with Potential Applications in Hemodialysis. Polymers 2022, 14, 148. https://doi.org/10.3390/polym14010148
Pandele AM, Oprea M, Dutu AA, Miculescu F, Voicu SI. A Novel Generation of Polysulfone/Crown Ether-Functionalized Reduced Graphene Oxide Membranes with Potential Applications in Hemodialysis. Polymers. 2022; 14(1):148. https://doi.org/10.3390/polym14010148
Chicago/Turabian StylePandele, Andreea Madalina, Madalina Oprea, Andreea Aura Dutu, Florin Miculescu, and Stefan Ioan Voicu. 2022. "A Novel Generation of Polysulfone/Crown Ether-Functionalized Reduced Graphene Oxide Membranes with Potential Applications in Hemodialysis" Polymers 14, no. 1: 148. https://doi.org/10.3390/polym14010148