Novel Organochlorinated Xerogels: From Microporous Materials to Ordered Domains
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
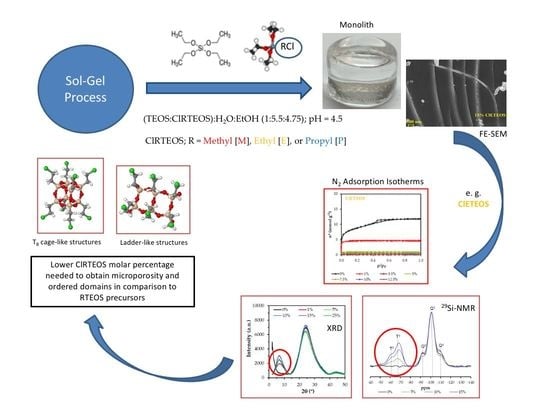
2.2. Synthesis of Silicon Hybrid Xerogels
2.3. Characterization of Silicon Hybrid Xerogels
3. Results
3.1. 29Si Nuclear Magnetic Resonance (NMR)
3.2. X-Ray Diffraction (XRD)
3.3. Skeletal Density
3.4. Porous Texture
3.4.1. Adsorption Isotherms and Textural Properties
3.4.2. Porosity Distribution
3.5. Field-Emission Scanning Electron Microscopy (FE-SEM)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Judeinstein, P.; Sanchez, C. Hybrid organic-inorganic materials: A land of multidisciplinarity. J. Mater. Chem. 1996, 6, 511–525. [Google Scholar] [CrossRef]
- Pastore, A.; Badocco, D.; Pastore, P. Influence of surfactant chain length, counterion and OrMoSil precursors on reversibility and working interval of pH colorimetric sensors. Talanta 2020, 212, 120739. [Google Scholar] [CrossRef] [PubMed]
- Gillanders, R.N.; Campbell, I.A.; Glackin, J.M.E.; Samuel, I.D.W.; Turnbull, G.A. Ormosil-coated conjugated polymers for the detection of explosives in aqueous environments. Talanta 2018, 179, 426–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Suslick, K.S. Ultrasonic Preparation of Porous Silica-Dye Microspheres: Sensors for Quantification of Urinary Trimethylamine N-Oxide. ACS Appl. Mater. Interfaces 2018, 10, 15820–15828. [Google Scholar] [CrossRef]
- Echeverría, J.C.; De Vicente, P.; Estella, J.; Garrido, J.J. A fiber-optic sensor to detect volatile organic compounds based on a porous silica xerogel film. Talanta 2012, 99, 433–440. [Google Scholar] [CrossRef]
- Echeverría, J.C.; Calleja, I.; Moriones, P.; Garrido, J.J. Fiber optic sensors based on hybrid phenyl-silica xerogel films to detect n-hexane: Determination of the isosteric enthalpy of adsorption. Beilstein J. Nanotechnol. 2017, 8, 475–484. [Google Scholar] [CrossRef] [Green Version]
- Niu, L.; Zhou, G.; Miao, X.; Yuan, X.; Kumar, R.; Liu, H.; Jiang, Y.; Zou, X.; Zhou, H.; Lü, H. Micro/Nanofiber with Hollow Silica Nanoparticles Thin-Film for Airborne Molecular Contaminants Real-Time Sensing. Hindawi Adv. Condens. Matter Phys. 2018, 2018, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Shamir, D.; Elias, I.; Albo, Y.; Meyerstein, D.; Burg, A. ORMOSIL-entrapped copper complex as electrocatalyst for the heterogeneous de-chlorination of alkyl halides. Inorg. Chim. Acta 2020, 500, 119225. [Google Scholar] [CrossRef]
- Ponamoreva, O.N.; Afonina, E.L.; Kamanina, O.A.; Lavrova, D.G.; Arlyapov, V.A.; Alferov, V.A.; Boronin, A.M. Yeast Debaryomyces hansenii within ORMOSIL Shells as a Heterogeneous Biocatalyst. Appl. Biochem. Microbiol. 2018, 54, 736–742. [Google Scholar] [CrossRef]
- Lin, W.; Zhang, X.; Cai, Q.; Yang, W.; Chen, H. Dehydrogenation-driven assembly of transparent and durable superhydrophobic ORMOSIL coatings on cellulose-based substrates. Cellulose 2020, 27, 7805–7821. [Google Scholar] [CrossRef]
- Liu, Z.; Tian, S.; Li, Q.; Wang, J.; Pu, J.; Wang, G.; Zhao, W.; Feng, F.; Qin, J.; Ren, L. Integrated Dual-Functional ORMOSIL Coatings with AgNPs@rGO Nanocomposite for Corrosion Resistance and Antifouling Applications. ACS Sustain. Chem. Eng 2020, 8, 6786–6797. [Google Scholar] [CrossRef]
- Scotland, K.M.; Shetranjiwalla, S.; Vreugdenhil, A.J. Curable hybrid materials for corrosion protection of steel: Development and application of UV-cured 3-methacryloxypropyltrimethoxysilane-derived coating. J. Coat. Technol. Res. 2020, 17, 977–989. [Google Scholar] [CrossRef]
- Bouvet-Marchand, A.; Graillot, A.; Abel, M.; Koudia, M.; Boutevin, G.; Loubat, C.; Grosso, D. Distribution of fluoroalkylsilanes in hydrophobic hybrid sol-gel coatings obtained by co-condensation. J. Mater. Chem. A. 2018, 6, 24899–24910. [Google Scholar] [CrossRef]
- Yue, D.; Feng, Q.; Huang, X.; Zhang, X.; Chen, H. In situ fabrication of a superhydrophobic ORMOSIL coating on wood by an ammonia-HMDS vapor treatment. Coatings 2019, 9, 556. [Google Scholar] [CrossRef] [Green Version]
- Malek, S.K.; Nodeh, H.R.; Akbari-Adergani, B. Silica-based magnetic hybrid nanocomposite for the extraction and preconcentration of some organophosphorus pesticides before gas chromatography. J. Sep. Sci. 2018, 41, 2934–2941. [Google Scholar] [CrossRef] [PubMed]
- Moriones, P.; Ríos, X.; Echeverría, J.C.; Garrido, J.J.; Pires, J.; Pinto, M. Hybrid organic-inorganic phenyl stationary phases for the gas separation of organic binary mixtures. Colloids Surfaces A Physicochem. Eng. Asp. 2011, 389, 69–75. [Google Scholar] [CrossRef]
- Zanut, A.; Palomba, F.; Scota, M.R.; Rebeccani, S.; Marcaccio, M.; Genovese, D.; Rampazzo, E.; valenti, G.; Paolucci, F.; Prodi, L. Dye-Doped Silica Nanoparticles for Enhanced ECL-Based Immunoassay. Angew. Chem. Int. Ed. 2020, 59, 21858–21863. [Google Scholar] [CrossRef]
- Meroni, D.; Ardizzone, S.; Cappelletti, G.; Ceotto, M.; Ratti, M.; Annunziata, R.; Benaglia, M.; Raimondi, L. Interplay between Chemistry and Texture in Hydrophobic TIO2 Hybrids. J. Phys. Chem. C 2011, 115, 18649–18658. [Google Scholar] [CrossRef]
- Alemán, J.; Chadwick, A.V.; He, J.; Hess, M.; Horie, K.; Jones, R.G.; Kratochvíl, P.; Meisel, I.; Mita, I.; Moad, G.; et al. Definitions of terms relating to the structure and processing of sols, gels, networks, and inorganic-organic hybrid materials (IUPAC recommendations 2007). Pure Appl. Chem. 2007, 79, 1801–1829. [Google Scholar] [CrossRef]
- Rios, X.; Moriones, P.; Echeverría, J.C.; Luquín, A.; Laguna, M.; Garrido, J.J. Characterisation of hybrid xerogels synthesised in acid media using methyltriethoxysilane (MTEOS) and tetraethoxysilane (TEOS) as precursors. Adsorption 2011, 17, 583–593. [Google Scholar] [CrossRef]
- Rios, X.; Moriones, P.; Echeverría, J.C.; Luquin, A.; Laguna, M.; Garrido, J.J. Ethyl group as matrix modifier and inducer of ordered domains in hybrid xerogels synthesised in acidic media using ethyltriethoxysilane (ETEOS) and tetraethoxysilane (TEOS) as precursors. Mater. Chem. Phys. 2013, 141, 166–174. [Google Scholar] [CrossRef]
- Moriones, P. Sintesis y Caracterización de Xerogeles Silíceos Híbridos (RTEOS/TEOS; R = P, Ph). Universidad Pública de Navarra: Pamplona, Spain, 2015. Available online: https://academica-e.unavarra.es/handle/2454/20351 (accessed on 14 November 2020).
- Chemtob, A.; Ni, L.; Croutxé-Barghorn, C.; Boury, B. Ordered Hybrids from Template-Free Organosilane Self-Assembly. Chem. Eur. J. 2014, 20, 1790–1806. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Shi, J. Chemistry of Mesoporous Organosilica in Nanotechnology: Molecularly Organic–Inorganic Hybridization into Frameworks. Adv. Mat. 2016, 28, 3235–3272. [Google Scholar] [CrossRef] [PubMed]
- Clara Gonçalves, M. Physisorption data for methyl-hybrid silica gels. J. Sol-Gel Sci. Technol. 2015, 75, 508–518. [Google Scholar] [CrossRef]
- Ramezani, M.; Vaezi, M.R.; Kazemzadeh, A. The influence of the hydrophobic agent, catalyst, solvent and water content on the wetting properties of the silica films prepared by one-step sol-gel method. Appl. Surf. Sci. 2015, 326, 99–106. [Google Scholar] [CrossRef]
- Estella, J.; Echeverría, J.C.; Laguna, M.; Garrido, J.J. Silica xerogels of tailored porosity as support matrix for optical chemical sensors. Simultaneous effect of pH, ethanol: TEOS and water: TEOS molar ratios, and synthesis temperature on gelation time, and textural and structural properties. J. Non. Cryst. Solids. 2007, 353, 286–294. [Google Scholar] [CrossRef]
- Estella, J.; Echeverría, J.C.; Laguna, M.; Garrido, J.J. Effects of aging and drying conditions on the structural and textural properties of silica gels. Microporous Mesoporous Mater. 2007, 102, 274–282. [Google Scholar] [CrossRef]
- Flores-López, S.L.; Villanueva, S.F.; Montes-Durán, M.A.; Cruz, G.; Garrido, J.J.; Arenillas, A. Advantages of microwave-assisted Synthesis of Silica Gels. Colloids Surf. A 2020, 604, 125248. [Google Scholar] [CrossRef]
- Yoshitake, H.; Kodate, T.; Takagi, T.; Kawamura, I.; naito, A. Polysilsesquioxanes with mixed self-assembled organic tethers Alkylchains and alkanoate–aminopropyl pairs. React. Funct. Polym. 2016, 99, 9–16. [Google Scholar] [CrossRef]
- Zacca, M.J.; Laurencin, D.; Richeter, S.; Clément, S.; Mehdi, A. New Layered Polythiophene-Silica Composite Through the Self-Assembly and Polymerization of Thiophene-Based Silylated Molecular Precursors. Molecules 2018, 23, 2510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ospino, I.; Luquin, A.; Jiménez-Ruiz, M.; Pérez-Landazábal, J.I.; Recarte, V.; Echeverría, J.C.; Laguna, M.; Urtasun, A.A.; Garrido, J.J. Computational Modeling and Inelastic Neutron Scattering Contributions to the Study of Methyl-silica Xerogels: A Combined Theoretical and Experimental Analysis. J. Phys. Chem. C 2017, 121, 22836–22845. [Google Scholar] [CrossRef]
- Tran, J.A.; Shea, K.J.; Loy, D.A. Methylene-bridged polysilsesquioxaes: Substitution of a methylene spacer within a silicate matrix. J. Mater. Sci. 2014, 49, 5006–5016. [Google Scholar] [CrossRef]
- Siriwong, C.; Sae-Oui, P.; Sirisinha, C. Comparison of coupling effectiveness among amino-, chloro-, and mercapto silanes in chloroprene rubber. Polym. Test. 2014, 38, 64–72. [Google Scholar] [CrossRef]
- Chen, G.; Zhou, Y.; Wang, X.; Li, J.; Xue, S.; Liu, Y.; Wang, Q.; Wang, J. Construction of porous cationic frameworks by crosslinking polyhedral oligomeric silsesquioxane units with N-heterocyclic linkers. Sci. Rep. 2015, 5, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Innocenzi, P. The Sol-Gel Transition, 2nd edSpringer: Sassari, Italy, 2019. [Google Scholar] [CrossRef]
- Musgo, J.; Echeverría, J.C.; Estella, J.; Laguna, M.; Garrido, J.J. Ammonia-catalyzed silica xerogels: Simultaneous effects of pH, synthesis temperature, and ethanol: TEOS and water: TEOS molar ratios on textural and structural properties. Microporous Mesoporous Mater. 2009, 118, 280–287. [Google Scholar] [CrossRef]
- Brinker, C.J.; Scherer, G.W. The Physics and Chemistry of Sol Gel Processing, 1st ed.; Academic Press Inc.: London, UK, 1990. [Google Scholar] [CrossRef]
- Kickelbick, G. Hybrid Materials Synthesis Characterization and Applications, 1st ed.Wiley—VCH Verlag GmbH & Co. KGaA: Wien, Austria, 2007. [Google Scholar] [CrossRef]
- Hall, C.J.; Ponnusamy, T.; Murphy, P.J.; Lindberg, M.; Antzutkin, O.N.; Griesser, H.J. A Solid-State Nuclear Magnetic Resonance Study of Post-Plasma Reactions in Organosilicone Microwave Plasma-Enhanced Chemical Vapor Deposition (PECVD) Coatings. ACS appl. Mater. Interfaces 2014, 6, 8353–8362. [Google Scholar] [CrossRef]
- Sakka, S. Handbook of Sol-Gel Science and Technology—Processing, Characterization and Application Volume II: Charact. Sol-Gel Mater. Prod., 1st ed.; Kluwer Academic Publishers: Norwell, MA, USA, 2005. [Google Scholar] [CrossRef]
- Rouquerol, F.; Rouquerol, J.; Sing, K.S.W.; Llewellyn, P.; Maurin, G. Adsorption by Powders and Porous Solids Principles, Methodology and Applications, 2nd ed.; Academic Press: San Diego, CA, USA, 1999. [Google Scholar] [CrossRef]
- Garrido, J.; Linares-solano, A.; Martìn-Martìnez, J.M.; Molina-Sabio, M.; Rodrìguez-Reinoso, F.; Torregrosa, R. Use of N2 vs: CO2 in the Characterization of Activated Carbons. Langmuir 1987, 3, 76–81. [Google Scholar] [CrossRef]
- García-Martínez, J.; Cazorla-Amorós, D.; Linares-Solano, A. Further evidences of the usefulness of CO2 adsorption to characterise microporous solids. Stud. Surf. Sci. Catal. 2000, 128, 485–494. [Google Scholar] [CrossRef]
- Torres-Luna, J.A.; Garriazo, J.G. Porous aluminosilicic solids obtained by thermal-acid modification of acommercial kaolinite-type natural clay. Solid State Sciences. 2019, 88, 29–35. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, Z. Structural Roles of Boron and Silicon in the CaOSiO2-B2O3 Glasses Using FTIR, Raman, and NMR Spectroscopy. Metall. and Mater. Trans. B. 2015, 46, 1549–1554. [Google Scholar] [CrossRef]
- El Felss, N.; Gharzouni, A.; Colas, M.; Cornette, J.; Sobrados, I.; Rossignol, S. Structural study of the effect of mineral additives on the transparency, stability, and aging of silicate gels. J. Sol-Gel Sci. Technol. 2020, 96, 265–275. [Google Scholar] [CrossRef]
- Park, E.S.; Ro, H.W.; Nguyen, C.V.; Jaffe, R.L.; Yoon, D.Y. Infrared spectroscopy study of microstructures of poly(silsesquioxane)s. Chem. Mater. 2008, 20, 1548–1554. [Google Scholar] [CrossRef]
- Uhlig, F.; Marsman, H.C. 29Si NMR—Some Practical Aspects. Gelest Inc.: Morrisville, PA, USA, 2008; pp. 208–222. Available online: http://www.gelest.com (accessed on 16 January 2021).
- Vasil’ev, S.G.; Volkov, V.I.; Tatarinova, E.A.; Muzafarov, A.M. A Solid-State NMR Investigation of MQ Silicone Copolymers. Appl. Magn. Reson. 2013, 44, 1015–1025. [Google Scholar] [CrossRef] [Green Version]
- Ivanovski, V.; Mayerhöfer, T.G.; Kriltz, A.; Popp, J. IR-ATR investigation of surface anisotropy in silicate glasses. Spectrochim. Acta—Part A Mol. Biomol. Spectrosc. 2017, 173, 608. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, J.Y.; Choi, W.; Lee, M.J.; Heo, J.; Choi, D.; Jung, S.; Kwon, J.; Choi, S.H.; Hong, J. Ladder-like polysilsesquioxanes with antibacterial chains and durable siloxane networks. Chem. Eng. J. 2020, 393, 124686. [Google Scholar] [CrossRef]
- Li, Q.; Guo, L.; Qiu, T.; Ye, J.; He, L.; Li, C.; Tuo, X. Polyurethane/polyphenylsilsequiloxane nanocomposite: From waterborne dispersions to coating films. Prog. Org. Coatings 2018, 122, 19–29. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Dudás, Z.; Len, A.; Ianăși, C.; Paladini, G. Structural modifications caused by the increasing MTES amount in hybrid MTES/TEOS-based silica xerogels. Mater. Charact. 2020, 167, 33–36. [Google Scholar] [CrossRef]
- Echeverría, J.C.; Estella, J.; Barbería, V.; Musgo, J.; Garrido, J.J. Synthesis and characterization of ultramicroporous silica xerogels. J. Non. Cryst. Solids. 2010, 356, 378–382. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, J.; Sun, W.; Xie, Q.R.; Xia, W.; Gu, H. Delivering hydrophilic and hydrophobic chemotherapeutics simultaneously by magnetic mesoporous silica nanoparticles to inhibit cancer cells. Int. J. Nanomed. 2012, 7, 999–1013. [Google Scholar] [CrossRef] [Green Version]








| Precursor | Precursor Molar Percentage (%) | 29Si RMN (ppm) | ||||
|---|---|---|---|---|---|---|
| T2 | T3 | Q2 | Q3 | Q4 | ||
| ClMTEOS | 5 | −68.5 | −76.6 | −92 | −100.9 | −110.4 |
| 10 | −68.9 | −76.8 | −91.8 | −101.0 | −110.1 | |
| 15 | −68.3 | −76.7 | −92.9 | −101.3 | −110.4 | |
| ClMTEOS | 5 | −60.2 | −67.7 | −91.7 | −100.8 | −109.7 |
| 10 | −60.3 | −68.8 | −91.7 | −100.8 | −109.7 | |
| 15 | −60.3 | −68.7 | −91.9 | −100.9 | −109.5 | |
| ClPTEOS | 5 | −57.0 | −65.0 | −91.6 | −100.8 | −110.3 |
| 10 | −57.4 | −65.5 | −91.9 | −100.7 | -109.6 | |
| MTEOS | 30 | −54.8 | −63.1 | −91.7 | −101.1 | −110.9 |
| 70 | −56.3 | −64.6 | a | −100.9 | −109.6 | |
| 100 | −57.0 | −65.8 | a | a | a | |
| ETEOS | 10 | −54.6 | −63.2 | −92.5 | −101.7 | −110.7 |
| 30 | −55.7 | −63.8 | −92.3 | −101.3 | −109.9 | |
| 60 | −56.4 | −65.0 | a | −101.9 | −110.0 | |
| PTEOS | 10 | −56.4 | −64.1 | −92.2 | −100.9 | −110.2 |
| 30 | −56.1 | −64.6 | −90.9 | −100.3 | −109.4 | |
| 60 | −56.8 | −65.7 | a | −101.4 | −110.4 | |
| Precursor | ClRTEOS | Peak 2θ < 10° | Peak 10° < 2θ < 30° | ||||
|---|---|---|---|---|---|---|---|
| Molar Percentage (%) | 2θ1 (°) | A1 | d1 (nm) | 2θ2 (°) | A2 | d2 (nm) | |
| TEOS | 0 | a | a | a | 24.16 | 68,812 | 0.368 |
| ClMTEOS | 1 | a | a | a | 24.26 | 66,451 | 0.367 |
| 5 | a | a | a | 24.30 | 65,847 | 0.366 | |
| 10 | a | a | a | 24.54 | 62,627 | 0.363 | |
| 15 | a | a | a | 24.42 | 60,377 | 0.365 | |
| 30 | 6.52 | 10,217 | 1.35 | 24.78 | 56,217 | 0.359 | |
| ClMTEOS | 1 | 7.17 | 8594 | 1.23 | 24.56 | 68,577 | 0.362 |
| 5 | 6.92 | 15,879 | 1.28 | 24.30 | 74,258 | 0.366 | |
| 10 | 6.76 | 16,568 | 1.31 | 24.14 | 81,785 | 0.369 | |
| 15 | 6.76 | 19,509 | 1.31 | 24.22 | 78,157 | 0.367 | |
| 25 | 6.76 | 15,780 | 1.31 | 23.90 | 71,722 | 0.372 | |
| ClPTEOS | 1 | a | a | a | 24.22 | 73,819 | 0.367 |
| 5 | a | a | a | 23.98 | 65,782 | 0.371 | |
| 10 | 5.80 | 5514 | 1.52 | 24.06 | 66,143 | 0.370 | |
| Precursor | ClRTEOS Molar Percentage (%) | aBET (N2) | aDR (CO2) | Vmicro (N2) | Vmicro (CO2) | Vmeso (N2) | Vtotal (N2) | BJH APS a | Ec (N2) b | Ec (CO2) b |
|---|---|---|---|---|---|---|---|---|---|---|
| (m2 g−1) | (cm3 g−1) | (nm) | (KJ mol−1) | |||||||
| TEOS | 0 | 697 | 510 | 0.283 | 0.195 | 0.074 | 0.407 | 3.61 | 15.27 | 19.71 |
| ClMTEOS | 1 | 700 | 465 | 0.289 | 0.178 | 0.210 | 0.560 | 4.21 | 14.27 | 19.77 |
| 3.5 | 691 | 475 | 0.285 | 0.182 | 0.061 | 0.394 | 3.55 | 14.82 | 18.93 | |
| 5 | 702 | 464 | 0.293 | 0.177 | 0.052 | 0.390 | 3.51 | 14.93 | 19.15 | |
| 7.5 | 693 | 457 | 0.288 | 0.175 | 0.036 | 0.364 | 3.41 | 15.26 | 18.98 | |
| 10 | 662 | 471 | 0.274 | 0.180 | 0.022 | 0.324 | 3.38 | 15.67 | 18.77 | |
| 15 | 591 | 428 | 0.248 | 0.164 | 0.013 | 0.278 | 3.30 | 15.81 | 18.82 | |
| 20 | 534 | 463 | 0.226 | 0.177 | 0.009 | 0.239 | 3.15 | 16.42 | 18.71 | |
| 25 | 422 | 381 | 0.175 | 0.146 | 0.002 | 0.174 | 2.56 | 18.32 | 18.29 | |
| 30 | 294 | 358 | 0.121 | 0.137 | 0.003 | 0.123 | 2.09 | 20.47 | 18.32 | |
| 35 | c | 347 | c | 0.132 | c | c | c | c | 18.04 | |
| ClMTEOS | 1 | 410 | 506 | 0.164 | 0.193 | 0.001 | 0.164 | 2.32 | 22.17 | 20.80 |
| 3.5 | c | 461 | c | 0.178 | c | c | c | c | 20.05 | |
| 5 | c | 451 | c | 0.172 | c | c | c | c | 20.64 | |
| 7.5 | c | 411 | c | 0.157 | c | c | c | c | 20.25 | |
| 10 | c | 371 | c | 0.142 | c | c | c | c | d | |
| 12.5 | c | 356 | c | 0.136 | c | c | c | c | 19.69 | |
| 15 | c | 428 | c | 0.164 | c | c | c | c | 19.05 | |
| 20 | c | 351 | c | 0.134 | c | c | c | c | 19.29 | |
| 25 | c | 345 | c | 0.132 | c | c | c | c | 18.55 | |
| ClPTEOS | 1 | 555 | 496 | 0.232 | 0.189 | 0.018 | 0.282 | 3.39 | 16.50 | 19.74 |
| 3.5 | 312 | 483 | 0.129 | 0.184 | 0.004 | 0.136 | 2.96 | 18.48 | 19.80 | |
| 5 | 318 | 434 | 0.129 | 0.167 | 0.004 | 0.136 | 2.85 | 19.98 | 19.49 | |
| 7.5 | 118 | 433 | 0.050 | 0.165 | 0.000 | 0.048 | 2.09 | 17.37 | 19.72 | |
| 10 | 132 | 359 | 0.056 | 0.137 | 0.000 | 0.053 | 2.18 | 15.72 | 17.69 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz-Quesada, G.; Espinal-Viguri, M.; López-Ramón, M.V.; Garrido, J.J. Novel Organochlorinated Xerogels: From Microporous Materials to Ordered Domains. Polymers 2021, 13, 1415. https://doi.org/10.3390/polym13091415
Cruz-Quesada G, Espinal-Viguri M, López-Ramón MV, Garrido JJ. Novel Organochlorinated Xerogels: From Microporous Materials to Ordered Domains. Polymers. 2021; 13(9):1415. https://doi.org/10.3390/polym13091415
Chicago/Turabian StyleCruz-Quesada, Guillermo, Maialen Espinal-Viguri, María Victoria López-Ramón, and Julián J. Garrido. 2021. "Novel Organochlorinated Xerogels: From Microporous Materials to Ordered Domains" Polymers 13, no. 9: 1415. https://doi.org/10.3390/polym13091415