Biodegradable Polyurethanes Based on Castor Oil and Poly (3-hydroxybutyrate)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
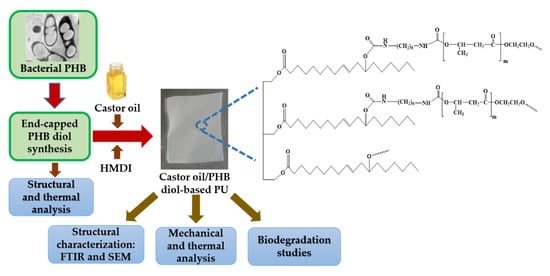
2.2. PHB Production and Extraction
2.3. Preparation of PHB Diol
2.4. Preparation of CO/PHBD-Based Polyurethane (CO/PHBD PU)
2.5. Analytical Procedures
2.5.1. Fourier Transform Infrared
2.5.2. Nuclear Magnetic Resonance
2.5.3. Molecular Weight Analysis
2.5.4. Hydroxyl Content Determination
2.5.5. Mechanical Analysis
2.5.6. Thermal Analysis
2.5.7. Scanning Electron Microscope
2.5.8. Solubility Test
2.5.9. Soil Biodegradation Test
3. Results and Discussion
3.1. PHB and PHBD Characterization
3.1.1. FTIR Analysis
3.1.2. 1H NMR Spectra
3.1.3. Molecular Weight Analysis of Synthesized PHBD
3.1.4. Thermal Properties of Synthesized PHB and PHBD
3.2. Structural and Morphological Characterization of CO/PHBD PU
3.2.1. FTIR Spectra
3.2.2. Morphological Analysis
3.3. Mechanical Analysis of CO/PHBD PU
3.3.1. Effect of NCO/OH Ratio
3.3.2. Effect of CO/PHBD Ratio
3.4. Thermal Analysis of CO/PHBD PU
3.4.1. Differential Scanning Calorimetry of CO/PHBD PU
3.4.2. Thermogravimetric Analysis of CO/PHBD PU
3.5. Solubility of CO/PHBD PU
3.6. Effect of Different Molecular Weight PHBD
3.6.1. Structural Analysis
3.6.2. Mechanical and Thermal Analysis
3.6.3. Soil Biodegradation Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tremblay-Parrado, K.K.; Bordin, C.; Nicholls, S.; Heinrich, B.; Donnio, B.; Averous, L. Renewable and responsive cross-linked systems based on polyurethane backbones from clickable biobased bismaleimide architecture. Macromolecules 2020, 53, 5869–5880. [Google Scholar] [CrossRef]
- Petrović, Z.S.; Yang, L.; Zlatanic, A.; Zhang, W.; Javni, I. Network structure and properties of polyurethanes from soybean oil. J. Appl. Polym. Sci. 2007, 105, 2717–2727. [Google Scholar] [CrossRef]
- Pfister, D.P.; Xia, Y.; Larock, R.C. Recent advances in vegetable oil-based polyurethanes. ChemSusChem 2011, 4, 703–717. [Google Scholar] [CrossRef]
- Pechar, T.W.; Sohn, S.; Wilkes, G.L.; Ghosh, S.; Frazier, C.E.; Fornof, A.; Long, T.E. Characterization and comparison of polyurethane networks prepared using soybean-based polyols with varying hydroxyl content and their blends with petroleum-based polyols. J. Appl. Polym. Sci. 2006, 101, 1432–1443. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Y.; Chen, R.; Kessler, M.R. Polyurethanes from solvent-free vegetable oil-based polyols. ACS Sustain. Chem. Eng. 2014, 2, 2465–2476. [Google Scholar] [CrossRef]
- Pantone, V.; Laurenza, A.G.; Annese, C.; Comparelli, R.; Fracassi, F.; Fini, P.; Nacci, A.; Russo, A.; Fusco, C.; D’Accolti, L. Preparation and characterization of soybean oil-based polyurethanes for digital doming applications. Materials 2017, 10, 848. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Liang, H.; Ziming, Y.; Teng, Y.; Ying, L.; Puwang, L.; Yang, Z.; Zhang, C. A solvent-free and scalable method to prepare soybean-oil-based polyols by thiol–ene photo-click reaction and biobased polyurethanes therefrom. ACS Sustain. Chem. Eng. 2017, 5, 7365–7373. [Google Scholar] [CrossRef]
- Tu, Y.-C.; Kiatsimkul, P.; Suppes, G.; Hsieh, F.H. Physical properties of water-blown rigid polyurethane foams from vegetable oil-based polyols. J. Appl. Polym. Sci. 2007, 105, 453–459. [Google Scholar] [CrossRef]
- Abdel Aziz, M.S.; Elsoholy, M.G.; Saad, G.R. Preparation and characterization of bio-based polyurethanes obtained from castor oil and poly (3-hydroxybutyrate) and their nanocomposites. Polym. Compos. 2018, 39, E489–E499. [Google Scholar] [CrossRef]
- Gurunathan, T.; Mohanty, S.; Nayak, S.K. Isocyanate terminated castor oil-based polyurethane prepolymer: Synthesis and characterization. Prog. Org. Coat. 2015, 80, 39–48. [Google Scholar] [CrossRef]
- Oprea, S.; Potolinca, V.O.; Gradinariu, P.; Joga, A.; Oprea, V. Synthesis, properties, and fungal degradation of castor-oil based polyurethane composites with different cellulose contents. Cellulose 2016, 23, 2515–2526. [Google Scholar] [CrossRef]
- Tang, B.-C.; Yao, C.-L.; Xieh, K.-Y.; Hong, S.-G. Improvement of physical properties of poly(glycerol sebacate) by copolymerization with polyhydroxybutyrate-diols. J. Polym. Res. 2017, 24, 215. [Google Scholar] [CrossRef]
- Wang, C.; Zheng, Y.; Sun, Y.; Fan, J.; Qin, Q.; Zhao, Z. A novel biodegradable polyurethane based on poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and poly(ethylene glycol) as promising biomaterials with the improvement of mechanical properties and hemocompatibility. Polym. Chem. 2016, 7, 6120. [Google Scholar] [CrossRef]
- Xue, D.; Fan, X.; Zhang, Z.; Lv, W. The synthesis of hydroxybutyrate-based block polyurethane from telechelic diols with robust thermal and mechanical properties. J. Chem. 2016, 2016, 9635165. [Google Scholar] [CrossRef] [Green Version]
- Naguib, H.F.; Abdel Aziz, M.S.; Sherif, S.M.; Saad, G.R. Synthesis and thermal characterization of poly(ester-ether urethane)s based on PHB and PCL-PEG-PCL blocks. J. Polym. Res. 2011, 18, 1217–1227. [Google Scholar] [CrossRef]
- Zhang, S.; Xiang, A.; Tian, H.; Rajulu, A.V. Water-blown castor oil-based polyurethane foams with soy protein as a reactive reinforcing filler. J. Polym. Environ. 2018, 26, 15–22. [Google Scholar] [CrossRef]
- Chen, J.-H.; Hu, D.-D.; Li, Y.-D.; Zhu, J.; Du, A.-K.; Zeng, J.-B. Castor oil-based high performance and reprocessable poly(urethane urea) network. Polym. Test. 2018, 70, 174–179. [Google Scholar] [CrossRef]
- Uscátegui, Y.L.; Arévalo, F.R.; Díaz, L.E.; Cobo, M.I.; Valero, M.F. Microbial degradation, cytotoxicity and antibacterial activity of polyurethanes based on modified castor oil and polycaprolactone. J. Biomater. Sci. Polym. Ed. 2016, 27, 1860–1879. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-H.; Hu, D.-D.; Li, Y.-D.; Meng, F.; Zhu, J.; Zeng, J.-B. Castor oil derived poly(urethane urea) networks with reprocessibility and enhanced mechanical properties. Polymer 2018, 143, 79–86. [Google Scholar] [CrossRef] [Green Version]
- Li, J.-W.; Cheng, Y.-H.; Lee, H.-T.; Wnag, C.-C.; Chiu, C.-W.; Suen, M.-C. Synthesis and properties of castor oil-based polyurethane containing short fluorinated segment. J. Appl. Polym. Sci. 2020, 137, e49062. [Google Scholar] [CrossRef]
- Tenorio-Alfonso, A.; Sánchez, M.C.; Franco, J.M. Synthesis and mechanical properties of bio-sourced polyurethane adhesives obtained from castor oil and MDI-modified cellulose acetate: Influence of cellulose acetate modification. Int. J. Adhes. Adhes. 2019, 95, 102404. [Google Scholar] [CrossRef]
- Khomlaem, C.; Aloui, H.; Kim, B.S. Biosynthesis of polyhydroxyalkanoates from defatted Chlorella biomass as an inexpensive substrate. Appl. Sci. 2021, 11, 1094. [Google Scholar] [CrossRef]
- Fiorese, M.L.; Freitas, F.; Pais, J.; Ramos, A.M.; de Aragão, G.M.F.; Reis, M.A.M. Recovery of polyhydroxybutyrate (PHB) from Cupriavidus necator biomass by solvent extraction with 1,2-propylene carbonate. Eng. Life Sci. 2009, 9, 454–461. [Google Scholar] [CrossRef]
- Saad, G.R. Blends of bacterial poly[(R)-3-hydroxybutyrate] with oligo[(R,S)-3-hydroxybutyrate]-diol. Polym. Int. 2002, 51, 338–348. [Google Scholar] [CrossRef]
- Yamanaka, K.; Kimura, Y.; Aoki, T.; Kudo, T. Effect of ethylene glycol on the end group structure of poly(3-hydroxybutyrate). Polym. Degrad. Stab. 2010, 95, 1284–1291. [Google Scholar] [CrossRef]
- Hirt, T.D.; Neuenschwander, P.; Suter, U.W. Telechelic diols from poly[(R)-3-hydroxybutyric acid] and Poly{[(R)-3-hydroxybutyric acid]-co-[(R)-3-hydroxyvaleric acid]}. Macromol. Chem. Phys. 1996, 197, 1609–1614. [Google Scholar] [CrossRef]
- Spitalsky, Z.; Lacık, I.; Lathova, E.; Janigova, I.; Chodak, I. Controlled degradation of polyhydroxybutyrate via alcoholysis with ethylene glycol or glycerol. Polym. Degrad. Stab. 2006, 91, 856–861. [Google Scholar] [CrossRef]
- Debuissy, T.; Pollet, E.; Averous, L. Synthesis and characterization of block poly(ester-ether-urethane)s from bacterial poly(3-hydroxybutyrate) oligomers. J. Polym. Sci. Part A-1 Polym. Chem. 2017, 55, 1949–1961. [Google Scholar] [CrossRef]
- Hong, S.-G.; Hsu, H.-W.; Ye, M.-T. Thermal properties and applications of low molecular weight polyhydroxybutyrate. J. Therm. Anal. Calorim. 2013, 111, 1243–1250. [Google Scholar] [CrossRef]
- Florian, P.; Jena, K.K.; Allauddin, S.; Narayan, R.; Raju, K.V.S.N. Preparation and characterization of waterborne hyperbranched polyurethane-urea and their hybrid coatings. Ind. Eng. Chem. Res. 2010, 49, 4517–4527. [Google Scholar] [CrossRef]
- Thakur, S.; Karak, N. Castor oil-based hyperbranched polyurethanes as advanced surface coating materials. Prog. Org. Coat. 2013, 76, 157–164. [Google Scholar] [CrossRef]
- Zhou, X.; Tu, W.; Hu, J. Preparation and characterization of two-component waterborne polyurethane comprised of water-soluble acrylic resin and HDI biuret. Chin. J. Chem. Eng. 2006, 14, 99–104. [Google Scholar] [CrossRef]
- Qiu, W.; Zhang, F.; Endo, T.; Hirotsu, T. Isocyanate as a compatibilizing agent on the properties of highly crystalline cellulose/polypropylene composites. J. Mater. Sci. 2005, 40, 3607–3614. [Google Scholar] [CrossRef]
- Pan, H.; Li, Z.; Yang, J.; Li, X.; Ai, X.; Hao, Y.; Zhang, H.; Dong, L. The effect of MDI on the structure and mechanical properties of poly(lactic acid) and poly(butylene adipate-co-butylene terephthalate) blends. RSC Adv. 2018, 8, 4610. [Google Scholar] [CrossRef] [Green Version]
- Xie, F.; Zhang, T.; Bryant, P.; Kurusingal, V.; Colwell, J.M.; Laycock, B. Degradation and stabilization of polyurethane elastomers. Prog. Polym. Sci. 2019, 90, 211–268. [Google Scholar] [CrossRef]
- Liu, K.L.; Choo, E.S.G.; Wong, S.Y.; Li, X.; He, C.B.; Wang, J.; Li, J. Designing poly[(R)-3-hydroxybutyrate]-based polyurethane block copolymers for electrospun nanofiber scaffolds with improved mechanical properties and enhanced mineralization capability. J. Phys. Chem. B 2010, 114, 7489–7498. [Google Scholar] [CrossRef] [PubMed]
- Hablot, E.; Zheng, D.; Bouquey, M.; Avérous, L. Polyurethanes based on castor oil: Kinetics, chemical, mechanical and thermal properties. Macromol. Mater. Eng. 2010, 293, 922–929. [Google Scholar] [CrossRef]
- Villegas-Villalobos, S.; Diaz, L.E.; Vilariño-Feltrer, G.; Vallés-Lluch, A.; Gómez-Tejedor, J.A.; Valero, M.F. Effect of an organotin catalyst on the physicochemical properties and biocompatibility of castor oil-based polyurethane/cellulose composites. J. Mater. Res. 2018, 33, 2598–2611. [Google Scholar] [CrossRef]
- Das, S.; Pandey, P.; Mohanty, S.; Nayak, S.K. Influence of NCO/OH and transesterified castor oil on the structure and properties of polyurethane: Synthesis and characterization. Mater. Express 2015, 5, 377–389. [Google Scholar] [CrossRef]
- Liao, W.-H.; Yang, S.-Y.; Wang, J.-Y.; Tien, H.-W.; Hsiao, S.-T.; Wang, Y.S.; Li, S.-M.; Ma, C.-C.M.; Wu, Y.-F. Effect of molecular chain length on the mechanical and thermal properties of amine-functionalized graphene oxide/polyimide composite films prepared by in situ polymerization. ACS Appl. Mater. Interfaces 2013, 5, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Shunmugam, R. Unraveling the effect of PEG chain length on the physical properties and toxicant removal capacities of cross-linked network synthesized by thiol−norbornene photoclick chemistry. ACS Omega 2020, 5, 2800–2810. [Google Scholar] [CrossRef]
- Hsu, S.-H.; Hsieh, C.-T.; Sun, Y.-M. Synthesis and characterization of waterborne polyurethane containing poly(3-hydroxybutyrate) as new biodegradable elastomers. J. Mater. Chem. B 2015, 3, 9089. [Google Scholar] [CrossRef]
- Gomez, E.F.; Luo, X.; Li, C.; Michel, F.C., Jr.; Li, Y. Biodegradability of crude glycerol-based polyurethane foams during composting, anaerobic digestion and soil incubation. Polym. Degrad. Stab. 2014, 102, 195–203. [Google Scholar] [CrossRef]
- Yeoh, F.H.; Lee, C.S.; Kang, Y.B.; Wong, S.F.; Cheng, S.F.; Ng, W.S. Production of biodegradable palm oil-based polyurethane as potential biomaterial for biomedical applications. Polymers 2020, 12, 1842. [Google Scholar] [CrossRef] [PubMed]
- Tai, N.L.; Adhikari, R.; Shanks, R.; Adhikari, B. Aerobic biodegradation of starch–polyurethane flexible films under soil burial condition: Changes in physical structure and chemical composition. Int. Biodeterior. Biodegrad. 2019, 145, 104793. [Google Scholar] [CrossRef]
- Abdel-Aziz, M.S.; Salama, H.E.; Sabaa, M.W. Biobased alginate/castor oil edible films for active food packaging. LWT 2018, 96, 455–460. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Liang, H.; Fang, C.; Zhang, C.; Luo, Y. Synthesis and properties of castor oil-based waterborne polyurethane/sodium alginate composites with tunable properties. Carbohydr. Polym. 2019, 298, 391–397. [Google Scholar] [CrossRef]

















| Reaction Time (h) | Mn of PHBD (g/mol) | ∆Hm (J/g) | Hydroxyl Content (mol) |
|---|---|---|---|
| 0 | - | 63.1 | - |
| 2 | 19233 ± 20 | 55.7 | 1.99 |
| 4 | 7675 ± 16 | 54.8 | 1.98 |
| 6 | 4817 ± 18 | 53.9 | 1.96 |
| 8 | 1890 ± 16 | 53.2 | 1.92 |
| CO/PHBD | ∆Hm (J/g) | Xc (%) | T10% (°C) | T50% (°C) | T90% (°C) |
|---|---|---|---|---|---|
| 1/0 | - | - | 301 | 379 | 534 |
| 9/1 | 1.00 | 6.87 | 305 | 377 | 475 |
| 8/2 | 2.89 | 9.91 | 295 | 371 | 474 |
| 7/3 | 4.65 | 10.6 | 285 | 361 | 468 |
| 6/4 | 8.13 | 14.0 | 272 | 344 | 481 |
| Sample | Tensile Strength (MPa) | Elongation at Break (%E) | T10% (°C) | T50% (°C) | T90% (°C) |
|---|---|---|---|---|---|
| CO/PHBD PU 2h | 10.8 ± 0.9 | 17.2 ± 0.8 | 288 | 353 | 446 |
| CO/PHBD PU 4h | 11.3 ± 1.2 | 18.1 ± 1.5 | 280 | 351 | 450 |
| CO/PHBD PU 6h | 12.1 ± 1.0 | 27.2 ± 1.8 | 275 | 348 | 468 |
| CO/PHBD PU 8h | 15.3 ± 1.0 | 28.8 ± 2.4 | 272 | 344 | 481 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saha, P.; Khomlaem, C.; Aloui, H.; Kim, B.S. Biodegradable Polyurethanes Based on Castor Oil and Poly (3-hydroxybutyrate). Polymers 2021, 13, 1387. https://doi.org/10.3390/polym13091387
Saha P, Khomlaem C, Aloui H, Kim BS. Biodegradable Polyurethanes Based on Castor Oil and Poly (3-hydroxybutyrate). Polymers. 2021; 13(9):1387. https://doi.org/10.3390/polym13091387
Chicago/Turabian StyleSaha, Pathikrit, Chanin Khomlaem, Hajer Aloui, and Beom Soo Kim. 2021. "Biodegradable Polyurethanes Based on Castor Oil and Poly (3-hydroxybutyrate)" Polymers 13, no. 9: 1387. https://doi.org/10.3390/polym13091387