A Review on Green Composites Based on Natural Fiber-Reinforced Polybutylene Succinate (PBS)
Abstract
:1. Introduction
2. PBS Synthesis, Structure, and Properties
3. History of Natural Fibers
4. Structure of Natural Fibers
5. The Concept of Natural Fiber/Biopolymer Green Composites
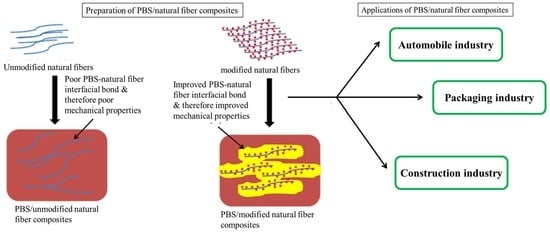
6. Preparation, Modification and Morphology
Natural Fiber/PBS Biopolymer Composites
7. Mechanical Properties of PBS/Natural Fiber Biocomposites
8. Biodegradation of PBS/Natural Fiber Composites
9. Specific Applications of the Natural Fiber-Reinforced PBS Composites and Their Natural Fiber PBS Blend Composites
10. Conclusions and Future Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dwivedi, P.; Mishra, P.K.; Mondal, M.K.; Srivastava, N. Non-biodegradable Polymeric Waste Pyrolysis for Energy. Heliyon 2019, 5, e02198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goda, K.; Cao, Y. Research and Development of Fully Green Composites Reinforced with Natural Fibers. J. Solid Mech. Mater. Eng. 2007, 1, 1073–1084. [Google Scholar] [CrossRef] [Green Version]
- Siakeng, R.; Jawaid, M.; Ariffin, H.; Sapuan, S.M.; Asim, M.; Saba, N. Natural Fiber Reinforced Polylactic Acid Composites. Rev. Polym. Compos. 2019, 40, 446–463. [Google Scholar] [CrossRef]
- Liu, L.F.; Yu, J.Y.; Cheng, L.D.; Qu, W.W. Mechanical Properties of Poly(Butylene Succinate)(PBS) Biocomposites Reinforced with Surface Modified Jute Fiber. Compos. Part A 2009, 40, 669–674. [Google Scholar] [CrossRef]
- Cunha, M.; Berthet, M.-A.; Pereira, R.; Covas, J.A.; Vicente, A.A.; Hilliou, L. Development of Polyhydroxyalkanoates/Beer Spent Grain Fibers Composites for Film Blowing Applications. Polym. Compos. 2015, 36, 1859–1865. [Google Scholar] [CrossRef]
- Kellersztein, I.; Amir, E.; Dotan, A. Grafting of Wheat Straw Fibers with Poly (Poly(ε-caprolactone) via Ring-opening Polymerization for Poly(Lactic Acid) Reinforcement. Polym. Adv. Technol. 2016, 27, 657–664. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, L.; Liang, D.; Xiao, W.; Lin, J. Mechanical and Thermal Properties of PLA Biocomposites Reinforced by Coir Fibers. Int. J. Polym. Sci. 2017, 2017, 1–8. [Google Scholar]
- Gironès, J.; López, J.P.; Mutjé, P.; Carvalho, A.J.F.D.; Curvelo, A.A.D.S.; Vilaseca, F. Natural Fiber-reinforced Thermoplastic Starch Composites Obtained by Melt Processing. Compos. Sci. Technol. 2012, 72, 858–863. [Google Scholar] [CrossRef]
- Mofokeng, J.P.; Luyt, A.S. Dynamic Mechanical Properties of PLA/PHBV, PLA/PCL, PHBV/PCL Blends and Their Nanocomposites with TiO2 as Nanofiller. Thermochim. Acta 2015, 613, 41–45. [Google Scholar] [CrossRef]
- Bin, T.; Qu, J.P.; Liu, L.M.; Feng, Y.H.; Hu, S.X.; Yin, X.C. Non-isothermal crystallization kinetics and dynamic mechanical thermal properties of poly (butylene succinate) composites reinforced with cotton stalk bast Fibers. Thermochim. Acta. 2011, 525, 141–149. [Google Scholar] [CrossRef]
- Torres, F.G.; Cubillas, M.L. Study of the Interfacial Properties of Natural Fiber Reinforced Polyethylene. Polym. Test. 2005, 24, 694–698. [Google Scholar] [CrossRef]
- Sanivada, U.K.; Mármol, G.; Brito, F.P.; Fangueiro, R. PLA Composites Reinforced with Flax and Jute Fibers–A Review of Recent Trends, Processing Parameters and Mechanical Properties. Polymers 2020, 12, 2373. [Google Scholar] [CrossRef]
- Keya, K.N.; Kona, N.A.; Koly, F.A.; Maraz, K.M.; Islam, M.N.; Khan, R.A. Natural Fiber Reinforced Polymer Composites: History, Types, Advantages and Applications. Mater. Eng. Res. 2019, 1, 69–85. [Google Scholar] [CrossRef] [Green Version]
- Bajpai, P.K.; Singh, I.; Madaan, J. Development and Characterization of PLA-based Green Composites: A Review. J. Thermoplast. Compos. Mater. 2012, 27, 52–81. [Google Scholar] [CrossRef]
- Dhakal, H.N.; Ismail, S.O.; Zhang, Z.; Barber, A.; Welsh, E.; Maigret, J.-E.; Beaugrand, J. Development of Sustainable Biodegradable Lignocellulosic Hemp Fiber/Polycaprolactone Biocomposites for Light Weight Applications. Compos. Part A 2018, 113, 350–358. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, L.; Ansari, M.N.M.; Pua, G.; Jawaid, M.; Islam, M. A Review on Natural Fiber Reinforced Polymer Composite and Its Applications. Int. J. Polym. Sci. 2015, 2015. [Google Scholar] [CrossRef] [Green Version]
- Shubhra, Q.T.; Alam, A.K.M.M.; Gafur, M.A.; Shamsuddin, S.M.; Khan, M.A.; Saha, M.; Saha, D.; Quaiyyum, M.A.; Khan, J.A.; Ashaduzzaman, M. Characterization of Plant and Animal Based Natural Fibers Reinforced Polypropylene Composites and Their Comparative Study. Fiber Polym. 2010, 11, 725–731. [Google Scholar] [CrossRef]
- Ramamoorthy, S.K.; Skrifvars, M.; Persson, A. A Review of Natural Fibers Used in Biocomposites: Plant, Animal and Regenerated Cellulose Fibers. Polym. Rev. 2015, 55, 107–162. [Google Scholar] [CrossRef]
- Faruk, O.; Bledzki, A.K.; Fink, H.-P.; Sain, M. Biocomposites Reinforced with Natural Fibers: 2000–2010. Prog. Polym. Sci. 2012, 37, 1552–1596. [Google Scholar] [CrossRef]
- Wang, L.; Toppinen, A.; Juslin, H. Use of Wood in Green Building: A Study of Expert Perspectives from the UK. J. Clean. Prod. 2014, 65, 350–361. [Google Scholar] [CrossRef]
- Thakur, K.; Kalia, S. Enzymatic Modification of Ramie Fibers and Its Influence on the Performance of Ramie-poly(Butylene Succinate) Biocomposites. Int. J. Plast. Technol. 2017, 21, 209–226. [Google Scholar] [CrossRef]
- Liu, D.Z.; Li, J.W.; Li, C.W.; Deng, Y.L.; Zhang, Z.Q.; Ye, Z.Y.; Zhu, S.M. Poly(Butylene Succinate)/Bamboo Powder Blends as Solid-phase Carbon Source and Biofilm Carrier for Denitrifying Biofilters Treating Wastewater from Recirculating Aquaculture System. Sci. Rep. 2018, 8, 3289. [Google Scholar] [CrossRef]
- Feng, Y.H.; Zhang, D.W.; Qu, J.P.; He, H.Z.; Xu, B.P. Rheological Properties of Sisal Fiber/Poly(Butylene Succinate) Composites. Polym. Test. 2011, 30, 124–130. [Google Scholar] [CrossRef]
- Xu, J.; Guo, B.H. Poly (Butylene Succinate) and Its Copolymers: Research, Development and Industrialization. Biotechnol. J. 2010, 5, 1149–1163. [Google Scholar] [CrossRef]
- Gowman, A.; Wang, T.; Rodriguez-Uribe, A.; Mohanty, A.K.; Misra, M. Bio-poly (Butylene Succinate) and Its Composites with Grape Pomace: Mechanical Performance and Thermal Properties. ACS Omega 2018, 3, 15205–15216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikolic, M.S.; Djonlagic, J. Synthesis and Characterization of Biodegradable Poly (Butylene Succinate-co-butylene Adipate) S. Polym. Degrad. Stab. 2001, 74, 263–270. [Google Scholar] [CrossRef]
- Tserki, V.; Matzinos, P.; Pavlidou, E.; Vachliotis, D.; Panayiotou, C. Biodegradable Aliphatic Polyesters. Part I. Properties and Biodegradation of Poly (Butylene Succinate-co-butylene Adipate). Polym. Degrad. Stab. 2006, 91, 367–376. [Google Scholar] [CrossRef]
- Han, Y.K.; Kim, S.R.; Kim, J. Preparation and Characterization of High Molecular Weight Poly (Butylene Succinate). Macromol. Res. 2002, 10, 108–114. [Google Scholar] [CrossRef]
- Nagata, M.; Goto, H.; Sakai, W.; Tsutsumi, N. Synthesis and Enzymatic Degradation of Poly (Tetramethylene Succinate) Copolymers with Terephthalic Acid. Polymer 2000, 41, 4373–4376. [Google Scholar] [CrossRef]
- Honda, N.; Taniguchi, I.; Miyamoto, M.; Kimura, Y. Reaction Mechanism of Enzymatic Degradation of Poly (butylene Succinate-co-terephthalate)(PBST) with a Lipase Originated from Pseudomonas cepacia. Macromol. Biosci. 2003, 3, 189–197. [Google Scholar] [CrossRef]
- Guo, B.H.; Ding, H.G.; Xu, X.L.; Xu, J.; Sun, Y.B. Studies on the Sequence Structure and Crystallinity of Poly (Butylene Succinate) Copolymers with Terephthalic Acid. Chem. J. Chin. U. 2003, 24, 2316–2319. [Google Scholar]
- Takasu, A.; Oishi, Y.; Iio, Y.; Inai, Y.; Hirabayashi, T. Synthesis of Aliphatic Polyesters by Direct Polyesterification of Dicarboxylic Acids with Diols under Mild Conditions Catalyzed by Reusable Rare-earth Triflate. Macromolecules 2003, 36, 1772–1774. [Google Scholar] [CrossRef]
- Park, J.W.; Kim, D.K.; Im, S.S. Crystallization Behaviour of Poly (Butylene Succinate) Copolymers. Polym. Int. 2002, 51, 239–244. [Google Scholar] [CrossRef]
- Chae, H.G.; Park, S.H.; Kim, B.C.; Kim, D.K. Effect of Methyl Substitution of the Ethylene Unit on the Physical Properties of Poly (Butylene Succinate). J. Polym. Sci. Part B Polym. Phys. 2004, 42, 1759–1766. [Google Scholar] [CrossRef]
- Sun, Y.B. Synthesis and Characterization of Biodegradable Poly (Butylenes Succinate) Copolymers. Master’s Thesis, Tsinghua University, Beijing, China, 2005. [Google Scholar]
- Sun, Y.; Xu, J.; Xu, Y.; Guo, B. Synthesis and Crystallization Behavior of Biodegradable Poly (Butylene Succinate-co-butylene Phenylsuccinate). Acta Polym. 2006, 6, 745. [Google Scholar] [CrossRef]
- Mochizuki, M.; Mukai, K.; Yamada, K.; Ichise, N.; Murase, S.; Iwaya, Y. Structural Effects upon Enzymatic Hydrolysis of Poly (Butylene Succinate-co-ethylene Succinate) S. Macromolecules 1997, 30, 7403–7407. [Google Scholar] [CrossRef]
- Cao, A.; Okamura, T.; Nakayama, K.; Inoue, Y.; Masuda, T. Studies on Syntheses and Physical Properties of Biodegradable Aliphatic Poly (Butylene Succinate-co-ethylene Succinate) S and Poly (Butylene Succinate-co-diethylene Glycol Succinate) S. Polym. Degrad. Stab. 2002, 78, 107–117. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z.; Bikiaris, D.N. Synthesis, Cocrystallization, and Enzymatic Degradation of Novel Poly (Butylene-co-propylene Succinate) Copolymers. Biomacromolecules 2007, 8, 2437–2449. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, J.; Guo, B.; Xie, X. Crystallization Kinetics and Morphology of Biodegradable Poly (Butylene Succinate-co-propylene Succinate) S. J. Polym. Sci. Part B Polym. Phys. 2007, 45, 420–428. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, J.; Liu, D.; Guo, B.; Xie, X. Synthesis and Characterization of Biodegradable Poly (Butylene Succinate-co-propylene Succinate) S. J. Appl. Polym. Sci. 2008, 109, 1881–1889. [Google Scholar] [CrossRef]
- Shaiju, P.; Dorian, B.B.; Senthamaraikannan, R.; Padamati, R.B. Biodegradation of Poly (Butylene Succinate)(PBS)/Stearate Modified Magnesium-Aluminium Layered Double Hydroxide Composites under Marine Conditions Prepared via Melt Compounding. Molecules 2020, 25, 5766. [Google Scholar] [CrossRef]
- Siracusa, V.; Lotti, N.; Munari, A.; Dalla Rosa, M. Poly (Butylene Succinate) and Poly (Butylene Succinate-co-adipate) for Food Packaging Applications: Gas Barrier Properties after Stressed Treatments. Polym. Degrad. Stab. 2015, 119, 35–45. [Google Scholar] [CrossRef]
- Fisher, C.H. History of Natural Fibers. J. Macromol. Sci. 1981, 15, 1345–1375. [Google Scholar] [CrossRef]
- Mwaikambo, L. Review of the History, Properties and Application of Plant Fibres. Afr. J. Sci. Technol. 2006, 7, 121. [Google Scholar]
- Mikuriya, T. Marijuana Medical Papers; Medi-Com Press: New York, NY, USA, 1973; pp. 13–27. [Google Scholar]
- Kozłowski, R.M.; Mackiewicz-Talarczyk, M. Introduction to Natural Textile Fibres. In Handbook of Natural Fibres; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–13. [Google Scholar]
- Thomas, S.; Paul, S.A.; Pothan, L.A.; Deepa, B. Natural Fibres: Structure, Properties and Applications. In Cellulose Fibers: Bio-and Nano-Polymer Composites; Springer: New York, NY, USA, 2011; pp. 3–42. [Google Scholar]
- Zakikhani, P.; Zahari, R.; Sultan, M.T.H.; Majid, D.L. Extraction and Preparation of Bamboo Fibre-reinforced Composites. Mater. Des. 2014, 63, 820–828. [Google Scholar] [CrossRef]
- Sanjay, M.R.; Arpitha, G.R.; Naik, L.L.; Gopalakrishna, K.; Yogesha, B. Applications of Natural Fibers and Its Composites: An Overview. Nat. Resour. 2016, 7, 108–114. [Google Scholar] [CrossRef] [Green Version]
- John, M.J.; Thomas, S. Biofibers and Biocomposites. Carbohydr. Polym. 2008, 71, 343–364. [Google Scholar] [CrossRef]
- Gowthaman, S.; Nakashima, K.; Kawasaki, S. A State-of-the-art Review on Soil Reinforcement Technology Using Natural Plant Fiber Materials: Past Findings, Present Trends and Future Directions. Materials 2018, 11, 553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kabir, M.M.; Wang, H.; Lau, K.T.; Cardona, F. Chemical Treatments on Plant-based Natural Fiber Reinforced Polymer Composites: An Overview. Compos. Part B Eng. 2012, 43, 2883–2892. [Google Scholar] [CrossRef]
- Rong, M.Z.; Zhang, M.Q.; Liu, Y.; Yang, G.C.; Zeng, H.M. The Effect of Fiber Treatment on the Mechanical Properties of Unidirectional Sisal-reinforced Epoxy Composites. Compos. Sci. Technol. 2001, 61, 1437–1447. [Google Scholar] [CrossRef]
- Jiang, X.; Bai, Y.; Chen, X.; Liu, W. A Review on Raw Materials, Commercial Production and Properties of Lyocell Fiber. J. Bioresour. Bioprod. 2020, 5, 16–25. [Google Scholar] [CrossRef]
- Bordoloi, S.; Garg, A.; Sreedeep, S. Potential of Uncultivated, Harmful and Abundant Weed as a Natural Geo-reinforcement Material. Adv. Civ. Eng. Mater. 2016, 5, 276–288. [Google Scholar] [CrossRef]
- Bordoloi, S.; Hussain, R.; Garg, A.; Sreedeep, S.; Zhou, W.H. Infiltration Characteristics of Natural Fiber Reinforced Soil. Transp. Geotech. 2017, 12, 37–44. [Google Scholar] [CrossRef]
- Mwaikambo, L.Y.; Ansell, M.P. Chemical Modification of Hemp, Sisal, Jute, and Kapok Fibers by Alkalization. J. Appl. Polym. Sci. 2002, 84, 2222–2234. [Google Scholar] [CrossRef]
- Kumar, R.; Obrai, S.; Sharma, A. Chemical Modifications of Natural Fiber for Composite Material. Der Chem. Sin. 2011, 2, 219–228. [Google Scholar]
- Yan, L.; Kasal, B.; Huang, L. A Review of Recent Research on the Use of Cellulosic Fibres, Their Fibre Fabric Reinforced Cementitious, Geo-polymer and Polymer Composites in Civil Engineering. Compos. Part B Eng. 2016, 92, 94–132. [Google Scholar] [CrossRef]
- Dittenber, D.B.; GangaRao, H.V. Critical Review of Recent Publications on Use of Natural Composites in Infrastructure. Compos. Part A Appl. Sci. Manuf. 2012, 43, 1419–1429. [Google Scholar] [CrossRef]
- John, M.J.; Anandjiwala, R.D. Recent Developments in Chemical Modification and Characterization of Natural Fiber-reinforced Composites. Polym. Compos. 2008, 29, 187–207. [Google Scholar] [CrossRef]
- Zwawi, M. A Review on Natural Fiber Bio-Composites; Surface Modifications and Applications. Molecules 2021, 26, 404. [Google Scholar] [CrossRef]
- De Backer, H. An Overview of the Characterization of Natural Cellulosic Fibers. Key Eng. Mater. 2021, 881, 107–116. [Google Scholar]
- Moshi, A.A.M.; Ravindran, D.; Bharathi, S.S.; Suganthan, V.; Singh, G.K.S. Characterization of New Natural Cellulosic Fibers—A Comprehensive Review. IOP Conf. Ser. Mater. Sci. Eng. 2019, 574, 1–12. [Google Scholar]
- Arthanarieswaran, V.P.; Kumaravel, A.; Saravanakumar, S.S. Characterization of New Natural Cellulosic Fiber from Acacia Leucophloea Bark. Int. J. Polym. Anal. 2015, 20, 367–376. [Google Scholar] [CrossRef]
- Sampathkumar, D.; Punyamurthy, R.; Bennehalli, B.; Venkateshappa, S.C. Physical Characterization of Natural Lignocellulosic Single Areca Fiber. Mater. Sci. Technol. 2015, 27, 121–135. [Google Scholar] [CrossRef]
- Feughelman, M. Mechanical Properties and Structure of Alpha-keratin Fibers: Wool, Human Hair and Related Fibers; UNSW Press: Sydney, Australia, 1997; pp. 1–159. [Google Scholar]
- Fakirov, S.; Bhattacharyya, D. Handbook of Engineering Biopolymers: Homopolymers. Blends, and Composites; Hanser Gardner: Cincinnati, OH, USA, 2007. [Google Scholar]
- Satyanarayana, K.G.; Arizaga, G.G.; Wypych, F. Biodegradable Composites Based on Lignocellulosic Fibers—An Overview. Prog. Polym. Sci. 2009, 34, 982–1021. [Google Scholar] [CrossRef]
- Li, X. Physical, Chemical, and Mechanical Properties of Bamboo and Its Utilization Potential for Fiberboard Manufacturing. Master’s Thesis, Louisiana State University, Baton Rouge, LA, USA, 2004; pp. 1–77. [Google Scholar]
- Okubo, K.; Fujii, T.; Yamamoto, Y. Development of Bamboo-based Polymer Composites and Their Mechanical Properties. Compos. Part A Appl. Sci. Manuf. 2004, 35, 377–383. [Google Scholar] [CrossRef]
- Reddy, N.; Yang, Y. Biofibers from Agricultural Byproducts for Industrial Applications. Trends Biotechnol. 2005, 23, 22–27. [Google Scholar] [CrossRef] [Green Version]
- Badrinath, R.; Senthilvelan, T. Comparative Investigation on Mechanical Properties of Banana and Sisal Reinforced Polymer Based Composites. Prog. Mater. Sci. 2014, 5, 2263–2272. [Google Scholar] [CrossRef] [Green Version]
- Cherian, B.M.; Leão, A.L.; De Souza, S.F.; Thomas, S.; Pothan, L.A.; Kottaisamy, M. Isolation of Nanocellulose from Pineapple Leaf Fibers by Steam Explosion. Carbohydr. Polym. 2010, 81, 720–725. [Google Scholar] [CrossRef]
- Laborel-Preneron, A.; Aubert, J.E.; Magniont, C.; Tribout, C.; Bertron, A. Plant Aggregates and Fibers in Earth Construction Materials: A Review. Constr. Build. Mater. 2016, 111, 719–734. [Google Scholar] [CrossRef]
- Lee, H.V.; Hamid, S.B.A.; Zain, S.K. Conversion of Lignocellulosic Biomass to Nanocellulose: Structure and Chemical Process. Sci. World J. 2014, 2014, 1–20. [Google Scholar] [CrossRef]
- Indran, S.; Raj, R.E.; Sreenivasan, V.S. Characterization of New Natural Cellulosic Fiber from Cissus Quadrangularis Stem. Carbohydr. Polym. 2015, 117, 392–399. [Google Scholar] [CrossRef]
- Ramesh, M.; Palanikumar, K.; Reddy, K.H. Plant Fiber Based Bio-composites: Sustainable and Renewable Green Materials. Renew. Sustain. Energy Rev. 2017, 79, 558–584. [Google Scholar] [CrossRef]
- Khalil, H.A.; Ismail, H.; Rozman, H.D.; Ahmad, M.N. The Effect of Acetylation on Interfacial Shear Strength between Plant Fibers and Various Matrices. Eur. Polym. J. 2001, 37, 1037–1045. [Google Scholar] [CrossRef]
- Lekha, K.R. Field Instrumentation and Monitoring of Soil Erosion in Coir Geotextile Stabilised Slopes—A Case Study. Geotext. Geomembr. 2004, 22, 399–413. [Google Scholar] [CrossRef]
- Baskaran, P.G.; Kathiresan, M.; Senthamaraikannan, P.; Saravanakumar, S.S. Characterization of New Natural Cellulosic Fiber from the Bark of Dichrostachys Cinerea. J. Nat. Fibers 2018, 15, 62–68. [Google Scholar] [CrossRef]
- Maheshwaran, M.V.; Hyness, N.R.J.; Senthamaraikannan, P.; Saravanakumar, S.S.; Sanjay, M.R. Characterization of Natural Cellulosic Fiber from Epipremnum aureum Stem. J. Nat. Fibers 2018, 15, 789–798. [Google Scholar] [CrossRef]
- Ramakrishna, G.; Sundararajan, T. Impact Strength of a Few Natural Fibre Reinforced Cement Mortar Slabs: A Comparative Study. Cement Concr. Comp. 2005, 27, 547–553. [Google Scholar] [CrossRef]
- Bordoloi, S.; Garg, A.; Sekharan, S. A Review of Physio-biochemical Properties of Natural Fibers and Their Application in Soil Reinforcement. Adv. Civ. Eng. Mater. 2017, 6, 323–359. [Google Scholar] [CrossRef]
- Naidu, A.L.; Raghuveer, D.; Suman, P. Studies on Characterization and Mechanical Behavior of Banana Peel Reinforced Epoxy Composites. Int. J. Sci. Eng. Res. 2013, 4, 844–851. [Google Scholar]
- Dhakal, H.N.; Zhang, Z.Y.; Richardson, M.O.W. Effect of Water Absorption on the Mechanical Properties of Hemp Fiber Reinforced Unsaturated Polyester Composites. Compos. Sci. Technol. 2007, 67, 1674–1683. [Google Scholar] [CrossRef]
- Hyness, N.R.J.; Vignesh, N.J.; Senthamaraikannan, P.; Saravanakumar, S.S.; Sanjay, M.R. Characterization of New Natural Cellulosic Fiber from Heteropogon Contortus Plant. J. Nat. Fibers 2018, 15, 146–153. [Google Scholar] [CrossRef]
- Athijayamani, A.; Thiruchitrambalam, M.; Natarajan, U.; Pazhanivel, B. Effect of Moisture Absorption on the Mechanical Properties of Randomly Oriented Natural Fibers/Polyester Hybrid Composite. Mater. Sci. Eng. A 2009, 517, 344–353. [Google Scholar] [CrossRef]
- Kozłowski, R.; Władyka-Przybylak, M. Flammability and Fire Resistance of Composites Reinforced by Natural Fibers. Polym. Adv. Technol. 2008, 19, 446–453. [Google Scholar] [CrossRef]
- Manikandan, N.; Morshed, M.N.; Karthik, R.; Al Azad, S.; Deb, H.; Rumi, T.M.; Ahmed, M.R. Improvement of Mechanical Properties of Natural Fiber Reinforced Jute/Polyester Epoxy Composite through Meticulous Alkali Treatment. Am. J. Org. Chem. 2017, 3, 9–18. [Google Scholar]
- Akil, H.M.; Cheng, L.W.; Ishak, Z.M.; Bakar, A.A.; Abd Rahman, M.A. Water Absorption Study on Pultruded Jute Fibre Reinforced Unsaturated Polyester Composites. Compos. Sci. Technol. 2009, 69, 1942–1948. [Google Scholar] [CrossRef]
- Joseph, S.; Oommen, Z.; Thomas, S. Environmental Durability of Banana-fiber-reinforced Phenol Formaldehyde Composites. J. Appl. Polym. Sci. 2006, 100, 2521–2531. [Google Scholar] [CrossRef]
- Kaddami, H.; Dufresne, A.; Khelifi, B.; Bendahou, A.; Taourirte, M.; Raihane, M.; Issartel, N.; Sautereau, H.; Gerard, J.F.; Sami, N. Short Palm Tree Fibers–Thermoset Matrices Composites. Compos. Part A Appl. Sci. Manuf. 2006, 37, 1413–1422. [Google Scholar] [CrossRef]
- Prithiviraj, M.; Muralikannan, R.; Senthamaraikannan, P.; Saravanakumar, S.S. Characterization of New Natural Cellulosic Fiber from the Perotis Indica Plant. Int. J. Polym. Anal. Charact. 2016, 21, 669–674. [Google Scholar] [CrossRef]
- Sharma, V.; Vinayak, H.K.; Marwaha, B.M. Enhancing Compressive Strength of Soil Using Natural Fibers. Constr Build Mater. 2015, 93, 943–949. [Google Scholar] [CrossRef]
- Yang, H.S.; Kim, H.J.; Park, H.J.; Lee, B.J.; Hwang, T.S. Effect of Compatibilizing Agents on Rice-husk Flour Reinforced Polypropylene Composites. Compos. Struct. 2007, 77, 45–55. [Google Scholar] [CrossRef]
- Gopinath, R.; Ganesan, K.; Saravanakumar, S.S.; Poopathi, R. Characterization of New Cellulosic Fiber from the Stem of Sida rhombifolia. Int. J. Polym. Anal. Charact. 2016, 21, 123–129. [Google Scholar] [CrossRef]
- Popelka, A.; Novák, I.; Al-Maadeed, M.A.S.; Ouederni, M.; Krupa, I. Effect of Corona Treatment on Adhesion Enhancement of LLDPE. Surf. Coat. Technol. 2018, 335, 118–125. [Google Scholar] [CrossRef]
- Kim, H.J. Effect of Water Absorption Fatigue on Mechanical Properties of Sisal Textile-reinforced Composites. Int. J. Fatigue. 2006, 28, 1307–1314. [Google Scholar] [CrossRef]
- Herrmann, A.S.; Nickel, J.; Riedel, U. Construction Materials Based upon Biologically Renewable Resources—From Components to Finished Parts. Polym. Degrad. Stab. 1998, 59, 251–261. [Google Scholar] [CrossRef]
- Feng, Y.; Shen, H.; Qu, J.; Liu, B.; He, H.; Han, L. Preparation and Properties of PBS/Sisal-fiber Composites. Polym. Eng. Sci. 2011, 51, 474–481. [Google Scholar] [CrossRef]
- Liu, L.; Yu, J.; Cheng, L.; Yang, X. Biodegradability of Poly(Butylene Succinate) (PBS) Composite Reinforced with Jute Fiber. Polym. Degrad. Stab. 2009, 94, 90–94. [Google Scholar] [CrossRef]
- Miao, X.; Lin, J.; Bian, F. Utilization of Discarded Crop Straw to Produce Cellulose Nanofibrils and Their Assemblies. J. Biores. Bioprod. 2020, 5, 26–36. [Google Scholar] [CrossRef]
- Li, H.; Liang, Y.; Li, P.; He, C. Conversion of Biomass Lignin to High-value Polyurethane: A Review. J. Biores. Bioprod. 2020, 5, 163–179. [Google Scholar] [CrossRef]
- Chen, Z.; Hu, J.; Ju, J.; Kuang, T. Fabrication of Poly (Butylene Succinate)/Carbon Black Nanocomposite Foams with Good Electrical Conductivity and High Strength by a Supercritical CO2 Foaming Process. Polymers 2019, 11, 1852. [Google Scholar] [CrossRef] [Green Version]
- Sair, S.; Oushabi, A.; Kammouni, A.; Tanane, O.; Abboud, Y.; Oudrhiri Hassan, F.; Laachachi, A.; El Bouari, A. Effect of Surface Modification on Morphological, Mechanical and Thermal Conductivity of Hemp Fiber: Characterization of the Interface of Hemp-Polyurethane Composite. Case Stud. Therm. Eng. 2017, 10, 550–559. [Google Scholar] [CrossRef]
- Sreekumar, P.A.; Thomas, S.P.; Saiter, J.M.; Joseph, K.; Unnikrishnan, G.; Thomas, S. Effect of Fiber Surface Modification on the Mechanical and Water Absorption Characteristics of Sisal/Polyester Composites Fabricated by Resin Transfer Molding. Compos. Part A Appl. Sci. Manuf. 2009, 40, 1777–1784. [Google Scholar] [CrossRef]
- Sreekumar, P.A.; Agoudjil, B.; Boudenne, A.; Unnikrishnan, G.; Ibos, L.; Fois, M.; Thomas, S. Transport Properties of Polyester Composite Reinforced with Treated Sisal Fibers. J. Reinf. Plast. Compos. 2012, 31, 117–127. [Google Scholar] [CrossRef]
- Cruz, J.; Fangueiro, R. Surface Modification of Natural Fibers: A Review. Procedia Eng. 2016, 155, 285–288. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.; Ghataura, A.; Takagi, H.; Haroosh, H.J.; Nakagaito, A.N.; Lau, K.-T. Polylactic Acid (PLA) with Coir Fibers: Evaluation of Mechanical Performance and Multifunctional Properties. Compos. Part A Appl. Sci. Manuf. 2014, 63, 76–84. [Google Scholar] [CrossRef] [Green Version]
- Siakeng, R.; Jawaid, M.; Asim, M.; Saba, N.; Saba, N.; Sanjay, M.R.; Siengchin, S.; Fouad, H. Alkali Treated Coir/Pineapple Leaf Fibers Reinforced PLA Hybrid Composites: Evaluation of Mechanical, Morphological, Thermal and Physical Properties. eXPRESS Polym. Lett. 2020, 14, 717–730. [Google Scholar] [CrossRef]
- Siakeng, R.; Jawaid, M.; Asim, M.; Siengchin, S. Accelerated Weathering and Soil Burial Effect on Biodegradability, Colour and Texture of Coir/Pineapple Leaf Fibers/PLA Biocomposites. Polymers 2020, 12, 458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haque, M.M.; Ali, M.E.; Hasan, M.; Islam, M.N.; Kim, H. Chemical Treatment of Coir Fiber Reinforced Polypropylene Composites. Ind. Eng. Chem. Res. 2012, 51, 3958–3965. [Google Scholar] [CrossRef]
- Orue, A.; Jauregi, A.; Unsuain, U.; Labidi, J.; Eceiza, A.; Arbelaiz, A. The Effect of Alkaline and Silane Treatments on Mechanical Properties and Breakage of Sisal Fibers and Poly(Lactic Acid)/Sisal Fiber Composites. Compos. Part A Appl. Sci. Manuf. 2016, 84, 186–195. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Y.; Chen, C. Synergistic Effects of Cellulose Nanocrystals and Alkali on the Mechanical Properties of Sisal Fibers and Their Bonding Properties with Epoxy. Compos. Part A Appl. Sci. Manuf. 2017, 101, 408–489. [Google Scholar] [CrossRef]
- Fiore, V.; Scalici, T.; Nicoletti, F.; Vitale, G.; Prestipino, M.; Valenza, A. A New Eco-friendly Chemical Treatment of Natural Fibers: Effect of Sodium Bicarbonate on Properties of Sisal Fiber and Its Epoxy Composites. Compos. Part B Eng. 2016, 85, 150–160. [Google Scholar] [CrossRef]
- Sun, Z. Progress in the Research and Applications of Natural Fiber-reinforced Polymer Matrix Composites. Sci. Eng. Compos. Mater. 2018, 25, 835–846. [Google Scholar] [CrossRef]
- Liu, H.Y.; Chen, F.Q.; Guo, R.B.; Zhang, G.; Qu, J. Effect of Compatibilizer on the Properties of PBS/Lignin Composites Prepared via a Vane Extruder. J. Polym. Eng. 2015, 35, 829–837. [Google Scholar] [CrossRef]
- Latif, R.; Wakeel, S.; Zaman Khan, N.; Noor Siddiquee, A.; Lal Verma, S.; Akhtar Khan, Z. Surface Treatments of Plant Fibers and Their Effects on Mechanical Properties of Fiber-reinforced Composites: A Review. J. Reinf. Plast. Compos. 2019, 38, 15–30. [Google Scholar] [CrossRef]
- Liminana, P.; Garcia-Sanoguera, D.; Quiles-Carrillo, L.; Balart, R.; Montanes, N. Development and Characterization of Environmentally Friendly Composites from Poly (Butylene Succinate)(PBS) and Almond Shell Flour with Different Compatibilizers. Compos. Part B Eng. 2018, 144, 153–162. [Google Scholar] [CrossRef]
- Yen, F.S.; Liao, H.T.; Wu, C.S. Characterization and Biodegradability of Agricultural Residue-filled Polyester Ecocomposites. Polym. Bull. 2013, 70, 1613–1629. [Google Scholar] [CrossRef]
- Zhou, M.; Li, Y.; He, C.; Jin, T.; Wang, K.; Fu, Q. Interfacial Crystallization Enhanced Interfacial Interaction of Poly(Butylene Succinate)/Ramie Fiber Biocomposites Using Dopamine as a Modifier. Compos. Sci. Technol. 2014, 91, 22–29. [Google Scholar] [CrossRef]
- Hong, G.; Cheng, H.; Zhang, S.; Rojas, O.J. Mussel-inspired Reinforcement of a Biodegradable Aliphatic Polyester with Bamboo Fibers. J. Clean. Prod. 2021, 296, 126587. [Google Scholar] [CrossRef]
- Zhao, L.; Huang, H.; Han, Q.; Yu, Q.; Lin, P.; Huang, S.; Yin, X.; Yang, F.; Zhan, J.; Wang, H.; et al. A Novel Approach to Fabricate Fully Biodegradable Poly (Butylene Succinate) Biocomposites Using a Paper-manufacturing and Compression Molding Method. Compos. Part A Appl. Sci. Manuf. 2020, 139, 106117. [Google Scholar] [CrossRef]
- Arabeche, K.; Abdelmalek, F.; Delbreilh, L.; Zair, L.; Berrayah, A. Physical and Rheological Properties of Biodegradable Poly(Butylene Succinate)/Alfa Fiber Composites. J. Thermoplast. Compos. Mater. 2020, 33, 1–19. [Google Scholar] [CrossRef]
- Calabia, B.P.; Ninomiya, F.; Yagi, H.; Oishi, A.; Taguchi, K.; Kunioka, M.; Funabashi, M. Biodegradable Poly(Butylene Succinate) Composites Reinforced by Cotton Fiber with Silane Coupling Agent. Polymers 2013, 5, 128–141. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Qiu, J.; Feng, H.; Zhang, M. The Interfacial Modification of Rice Straw Fiber Reinforced Poly(Butylene Succinate) Composites: Effect of Amino Silane with Different Alkoxy Groups. J. Appl. Polym. Sci. 2012, 125, 3211–3220. [Google Scholar] [CrossRef]
- Liang, Z.; Pan, P.; Zhu, B.; Dong, T.; Inoue, Y. Mechanical and Thermal Properties of Poly(Butylene Succinate)/Plant Fiber Biodegradable Composite. J. Appl. Polym. Sci. 2010, 115, 3559–3567. [Google Scholar] [CrossRef]
- Soatthiyanon, N.; Aumnate, C.; Srikulkit, K. Rheological, Tensile, and Thermal Properties of Poly(Butylene Succinate) Composites Filled with Two Types of Cellulose (Kenaf Cellulose Fiber and Commercial Cellulose). Polym. Compos. 2020, 41, 2777–2791. [Google Scholar] [CrossRef]
- Frollini, E.; Bartolucci, N.; Sisti, L.; Celli, A. Poly(Butylene Succinate) Reinforced with Different Lignocellulosic Fibers. Ind. Crops Prod. 2013, 45, 160–169. [Google Scholar] [CrossRef]
- Han, Q.; Zhao, L.; Lin, P.; Zhu, Z.; Nie, K.; Yang, F.; Wang, L. Poly(Butylene Succinate) Biocomposite Modified by Amino Functionalized Ramie Fiber Fabric Towards Exceptional Mechanical Performance and Biodegradability. React. Funct. Polym. 2020, 146, 104443. [Google Scholar] [CrossRef]
- Sisti, L.; Kalia, S.; Torato, G.; Vannini, M.; Negroni, A.; Zanaroli, G.; Celli, A. Enzymatically Treated Curaua Fibers in Poly(Butylene Succinate)-based Biocomposites. J. Environ. Chem. Eng. 2018, 6, 4452–4458. [Google Scholar] [CrossRef]
- Xu, X.L.; Zhang, M.; Qiang, Q.; Song, J.Q.; He, W.Q. Study on the Performance of the Acetylated Bamboo Fiber/PBS Composites by Molecular Dynamics Simulation. J. Compos. Mater. 2016, 50, 995–1003. [Google Scholar] [CrossRef]
- Nam, T.H.; Ogihara, S.; Nakatani, H.; Kobayashi, S.; Song, J. Mechanical and Thermal Properties and Water Absorption of Jute Fiber Reinforced Poly(Butylene Succinate) Biodegradable Composites. Adv. Compos. Mater. 2012, 21, 241–258. [Google Scholar] [CrossRef]
- Wu, C.-S.; Liao, H.-T.; Jhang, J.-J. Palm Fiber-reinforced Hybrid Composites of Poly(Butylene Succinate): Characterisation and Assessment of Mechanical and Thermal Properties. Polym. Bull. 2013, 70, 3443–3462. [Google Scholar] [CrossRef]
- Zhou, M.; Yan, J.; Li, Y.; Geng, C.; He, C.; Wang, K.; Fu, Q. Interfacial Strength and Mechanical Properties of Biocomposites Based on Ramie Fibers and Poly(Butylene Succinate). RSC Adv. 2013, 3, 26418–26426. [Google Scholar] [CrossRef]
- Frollini, E.; Bartolucci, N.; Sisti, L.; Celli, A. Biocomposites Based on Poly(Butylene Succinate) and Curaua: Mechanical and Morphological Properties. Polym. Test. 2015, 45, 168–173. [Google Scholar] [CrossRef]
- Then, Y.Y.; Ibrahim, N.A.; Zainuddin, N.; Ariffin, H.; Yunus, W.M.Z.W. Oil Palm Mesocarp as New Lignocellulosic Material for Fabrication of Polymer/Fiber Biocomposites. Int. J. Polym. Sci. 2013, 2013, 1–7. [Google Scholar] [CrossRef]
- Nakayama, A.; Yamano, N.; Kawasaki, N. Biodegradation in Seawater of Aliphatic Polyesters. Polym. Degrad. Stab. 2019, 166, 290–299. [Google Scholar] [CrossRef]
- Sekiguchi, T.; Saika, A.; Nomura, K.; Watanabe, T.; Watanabe, T.; Fujimoto, Y.; Enoki, M.; Sato, T.; Kato, C.; Kanehiro, H. Biodegradation of Aliphatic Polyesters Soaked in Deep Seawaters and Isolation of Poly (ε-caprolactone)-degrading Bacteria. Polym. Degrad. Stab. 2011, 96, 1397–1403. [Google Scholar] [CrossRef]
- Sashiwa, H.; Fukuda, R.; Okura, T.; Sato, S.; Nakayama, A. Microbial Degradation Behavior in Seawater of Polyester Blends Containing Poly (3-hydroxybutyrate-co-3-hydroxyhexanoate)(PHBHHx). Mar. Drugs. 2018, 16, 34. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.; Qian, L.; Yin, Q.; Yu, N.; Liu, T.; Tian, D. Biodegradability Studies of Poly (Butylene Succinate) Composites Filled with Sugarcane Rind Fiber. Polym. Test. 2018, 66, 319–326. [Google Scholar] [CrossRef]
- Peças., P.; Carvalho, H.; Salman, H.; Leite, M. Natural Fiber Composites and Their Applications: A Review. J. Compos. Sci. 2018, 2, 66. [Google Scholar] [CrossRef] [Green Version]
- Holmes, M. Biocomposites Take Natural Step Forward: Applications for Biocomposites and the Use of Natural Fiber Reinforcements are Increasing. Reinforced Plastics Looks at a Number of Examples. Reinf. Plast. 2019, 63, 194–201. [Google Scholar] [CrossRef]
- Su, S.; Kopitzky, R.; Tolga, S.; Kabasci, S. Polylactide (PLA) and Its Blends with Poly (Butylene Succinate)(PBS): A Brief review. Polymers 2019, 11, 1193. [Google Scholar] [CrossRef] [Green Version]
- Huang, A.; Peng, X.; Geng, L.; Zhang, L.; Huang, K.; Chen, B.; Gu, Z.; Kuang, T. Electrospun Poly (Butylene Succinate)/Cellulose Nanocrystals Bio-nanocomposite Scaffolds for Tissue Engineering: Preparation, Characterization and in vitro Evaluation. Polym. Test. 2018, 71, 101–109. [Google Scholar] [CrossRef]
- Chaari, R.; Khlif, M.; Mallek, H.; Bradai, C.; Lacoste, C.; Belguith, H.; Tounsi, H.; Dony, P. Enzymatic Treatments Effect on the Poly (Butylene Succinate)/Date Palm Fibers Properties for Bio-composite Applications. Ind. Crop. Prod. 2020, 148, 112270. [Google Scholar] [CrossRef]
- Ayu, R.S.; Khalina, A.; Harmaen, A.S.; Zaman, K.; Nurrazi, N.M.; Isma, T.; Lee, C.H. Effect of Empty Fruit Brunch Reinforcement in Polybutylene-succinate/Modified Tapioca Starch Blend for Agricultural Mulch Films. Sci. Rep. 2020, 10, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vorawongsagul, S.; Pratumpong, P.; Pechyen, C. Preparation and Foaming Behavior of Poly (Lactic Acid)/Poly (Butylene Succinate)/Cellulose Fiber Composite for Hot Cups Packaging Application. Food Packag. Shelf Life 2021, 27, 100608. [Google Scholar] [CrossRef]
- Saeed, U.; Nawaz, M.A.; Al-Turaif, H.A. Wood Flour Reinforced Biodegradable PBS/PLA Composites. J. Compos. Mater. 2018, 52, 2641–2650. [Google Scholar] [CrossRef]
- Dash, B.N.; Nakamura, M.; Sahoo, S.; Kotaki, M.; Nakai, A.; Hamada, H. Mechanical Properties of Hemp Reinforced Poly (Butylene Succinate) Biocomposites. J. Biobased Mater. 2008, 2, 273–281. [Google Scholar] [CrossRef]
- Domínguez-Robles, J.; Larrañeta, E.; Fong, M.L.; Martin, N.K.; Irwin, N.J.; Mutjé, P.; Tarrés, Q.; Delgado-Aguilar, M. Lignin/Poly (Butylene Succinate) Composites with Antioxidant and Antibacterial Properties for Potential Biomedical Applications. Int. J. Biol. Macromol. 2020, 145, 92–99. [Google Scholar] [CrossRef]
- Platnieks, O.; Barkane, A.; Ijudina, N.; Gaidukova, G.; Thakur, V.K.; Gaidukovs, S. Sustainable Tetra Pak Recycled Cellulose/Poly (Butylene Succinate) Based Woody-like Composites for a Circular Economy. J. Clean. Prod. 2020, 270, 122321. [Google Scholar] [CrossRef]
- Joy, J.; Jose, C.; Yu, X.; Mathew, L.; Thomas, S.; Pilla, S. The Influence of Nanocellulosic Fiber, Extracted from Helicteres isora, on Thermal, Wetting and Viscoelastic Properties of Poly (Butylene Succinate) Composites. Cellulose 2017, 24, 4313–4323. [Google Scholar] [CrossRef]
- Platnieks, O.; Gaidukovs, S.; Barkane, A.; Sereda, A.; Gaidukova, G.; Grase, L.; Thakur, V.K.; Filipova, I.; Fridrihsone, V.; Skute, M.; et al. Bio-based Poly (Butylene Succinate)/Microcrystalline Cellulose/Nanofibrillated Cellulose-based Sustainable Polymer Composites: Thermo-mechanical and Biodegradation Studies. Polymers 2020, 12, 1472. [Google Scholar] [CrossRef]
- Ju, J.; Gu, Z.; Liu, X.; Zhang, S.; Peng, X.; Kuang, T. Fabrication of Bimodal Open-porous Poly (Butylene Succinate)/Cellulose Nanocrystals Composite Scaffolds for Tissue Engineering Application. Int. J. Biol. Macromol. 2020, 147, 1164–1173. [Google Scholar] [CrossRef]






















| Type of Fiber | World Production (103 Ton) per Year |
|---|---|
| Abaca | 70.00 |
| Bamboo | 30,000.00 |
| Caraua | >1.00 |
| Coir | 100.00 |
| Cotton | 25,000.00 |
| Flax | 830.00 |
| Grass | 700.00 |
| Hemp | 214.00 |
| Jute | 2300.00 |
| Kenaf | 970.00 |
| Oil palm | 40.00 |
| Pineapple | 74.00 |
| Ramie | 100.00 |
| Sisal | 378.00 |
| Sugar cane bagasse | 75,000.00 |
| Physical Properties | PBS | PP | HDPE | LDPE |
|---|---|---|---|---|
| Glass transition temperature (°C) | −32 | −5 | −120 | −120 |
| Melting temperature (°C) | 114 | 163 | 129 | 110 |
| Heat distortion temperature (°C) | 97 | 110 | 82 | 49 |
| Tensile strength (MPa) | 34 | 33 | 28 | 10 |
| Elongation at break (%) | 560 | 415 | 700 | 300 |
| Izod impact strength (J/m) | 300 | 20 | 40 | >400 |
| Degree of crystallinity (%) | 35–45 | 56 | 69 | 49 |
| Natural Fiber | Cellulose Content (%) | Hemicellulose Content (%) | Lignin Content (%) | Pectin Content (%) | Wax Content (%) | Ash Content (%) | Moisture Content (%) | Refs |
|---|---|---|---|---|---|---|---|---|
| Abaca | 56–63 | 20–25 | 7–12 | 0.8 | 3 | - | - | [19,59,60,61,62,63,64] |
| Acacia Arabica (Indian gum Arabic tree) | 68.10 | 9.36 | 16.86 | - | 0.49 | - | - | [65] |
| Acacia Leucophloea (White-barked acacia) | 68.09 | 13.6 | 17.73 | - | 0.55 | 0.08 | 8.83 | [65,66] |
| Acacia Planifrons (Umbrella thorn) | 73.1 | 9.41 | 12.04 | - | 0.57 | 4.06 | 8.21 | [65] |
| Agave | 68.42 | 4.85 | 4.85 | - | 0.26 | - | 7.69 | [65] |
| Alfa | 45.4 | 38.5 | 14.9 | - | 2 | - | - | [59,60,61,63,64] |
| Areca | 57.35–58.21 | 13–15.42 | 23–24 | - | 0.12 | - | - | [63,67,68] |
| Bagasse | 32–55.2 | 16.8–25 | 19–25.3 | 10 | - | - | - | [52,59,60,63,64,69,70] |
| Bamboo | 26–55 | 20.5–30 | 15–32.2 | - | - | - | - | [19,49,52,59,60,63,64,71,72] |
| Banana | 60–65 | 12.5–25 | 5–10 | 4 | - | - | - | [52,59,63,64,69,70,73,74,75,76] |
| Barley | 31–45 | 27–38 | 8–19 | - | 2–7 | - | - | [52,63,73,77] |
| Cissus Quadrangularis (veld grape) root | 77.17 | 11.02 | 10.45 | - | 0.14 | - | 7.3 | [65] |
| Cissus Quadrangularis (veld grape) stem | 82.73 | 7.96 | 11.27 | - | 0.18 | - | 6.6 | [65,78] |
| Coir | 32–45.6 | 0.15–21 | 40–45 | 4 | - | - | - | [52,57,59,60,61,63,64,79,80,81] |
| Corn | 38–40 | 28 | 7–21 | - | 3.6–7 | - | - | [63,73] |
| Cotton | 82.7–90 | 4–5.7 | 0.75 | 6 | 0.6 | - | - | [59,60,61,63,64] |
| Curaua | 70.7–73.6 | 9.9 | 7.5–11.1 | - | - | - | - | [19,59,60,61,63,64,69] |
| Dichrostachys Cinerea (sicklebush) | 72.4 | 13.08 | 16.89 | - | 0.57 | 3.97 | 9.82 | [65,82] |
| Epipremnum Aureum (Devil’s ivy) | 66.34 | 13.42 | 14.01 | - | 0.37 | 4.61 | 7.41 | [65,83] |
| Eucalyptus | 41.7 | 32.56 | 25.4 | 8.2 | 0.22 | - | - | [63,84] |
| Flax | 62–81 | 4–20.6 | 2.2–5 | 0.9 | 1.5–1.7 | - | 10 | [19,52,59,60,63,64,65,69,70,85] |
| Furcraea Foetida | 68.35 | 11.46 | 12.32 | - | 0.24 | 6.53 | 5.43 | [65] |
| Hemp | 67–81 | 5.5–22 | 2.9–13 | 0.8–0.9 | 0.8–2.3 | - | 10.8 | [19,52,59,60,63,64,65,86,87] |
| Henequen | 60–77.6 | 28 | 8–13.1 | - | 0.5 | - | - | [59,60,61,63,64] |
| Heteropogon Contortus (Spear grass) | 64.84 | 19.34 | 13.56 | - | 0.22 | - | 7.4 | [65,88] |
| Hibiscus | 28 | 25 | 22.7 | - | - | - | - | [63,89] |
| Isora | 74 | - | 23 | - | 1.1 | - | - | [59,60,63,64] |
| Jute | 56–72 | 12–35 | 9–14 | 0.2 | 0.5 | 1 | 12.6 | [19,52,57,59,60,63,64,65,70,90,91,92] |
| Kenaf | 53.14–53.5 | 3–33 | 8.18–21.5 | 2 | - | 3.5 | 9 | [52,59,60,63,64,65,76,79,93] |
| Kudzu | 33 | 11.6 | 14 | - | - | - | - | [59,64] |
| Nettle | 86 | 10 | - | - | 4 | - | - | [59,64] |
| Oil Palm | 65 | - | 29 | - | - | - | - | [59,64] |
| Palm | 32–35.8 | 24.1–28.1 | 26.5–28.9 | - | - | - | - | [52,94] |
| Perotis indica (Indian comet grass) | 68.4 | 15.7 | 8.35 | - | 0.32 | 4.32 | 9.54 | [65,95] |
| Phromium | 67 | 30 | 11 | - | - | - | - | [60,63] |
| Piassava | 28.6 | 25.8 | 45 | - | - | - | - | [59,64] |
| Pine | 67.29 | 67.29 | 11.57 | - | - | - | - | [52,96] |
| Pineapple | 80.5-81 | 17.5 | 8.3–12.7 | 4 | - | - | - | [19,59,60,63,64] |
| Prosopis Juliflora (Mesquite) | 61.65 | 16.14 | 17.11 | - | 0.61 | 5.2 | 9.48 | [65] |
| Ramie | 72 | 5–16.7 | 0.6–0.8 | 2 | 0.3 | - | - | [59,60,63,64,86] |
| Red Banana Penduncle | 72.90 | 11.01 | 15.99 | - | 0.32 | 2.79 | 9.36 | [65] |
| Rice | 59.9 | 59.9 | 20.6 | - | - | - | - | [52,97] |
| Rice husk | 28–36 | 23–28 | 12–14 | - | 14–20 | - | - | [63,89] |
| Sida Rhombifolia (arrowleaf sida) stem | 75.09 | 15.43 | 7.48 | - | 0.49 | 4.07 | 12.02 | [65,98] |
| Sisal | 57–73 | 11.5–16 | 8–12 | 1.2 | 2 | - | 17 | [19,52,59,60,63,64,65,72,99,100] |
| Sorghum | 27 | 25 | 11 | - | - | - | - | [63,73] |
| Sponge Gourd | 63 | 19.4 | 11.2 | - | 3 | - | - | [59,64] |
| Straw (wheat) | 38–45 | 15–31 | 12–20 | - | - | - | - | [59,64] |
| Sun Hemp | 41–48 | 8.3–13 | 22.7 | - | - | - | - | [59,64] |
| Water Hyacith | 43.58–47.38 | 19.77–22.23 | 9.52–13.08 | - | - | - | - | [52,56,57,85] |
| Wheat | 30–38 | 26–50 | 15–19 | - | 6.8 | - | - | [52,63,77,84] |
| PBS/Natural Fiber Composites | Preparation Methods | Modification | Summary of the Results | Refs |
|---|---|---|---|---|
| PBS/alfa fiber | Compression molding method | Alkaline treatment | Fiber alkaline treatment resulted in:
| [126] |
| PBS/Cotton fiber | Compression molding at 150 °C for 5 min | Silane treatment |
| [127] |
| PBS/rice straw fiber | Injection molding | Amino silane treated | Treatment of fibers with 3-(2-aminoethylaminopropyl)-trimethoxysilane (AEAPTMES) achieved the best interfacial bonding results. | [128] |
| PBS/Kenaf fiber (KF) | Melt mixing method | ---------------- | Fibers were embedded in the PBS matrix; however, there was a phase separation between kenaf and PBS. | [129] |
| PBS/Cellulose fiber (Cellulose extracted from kenaf fiber and commercial cellulose) | Melt mixing at 200 °C for 5 min | Hydrochloric acid (HCl) and sodium hydroxide (NaOH) treatment |
| [130] |
| PBS/lignocellulosic fibers (coconut, sugarcane bagasse, curaua, sisal) | Thermo-pressing mold method | Ethanol and cyclohexane extraction process |
| [131] |
| PBS/ramie fiber Fabric (RFF) | Thermal compressive process | Treatment with 2% of 3 Triethoxysilylpropylamine (KH550) |
| [132] |
| PBS/curaua fibers | Compression molding | Enzymatic treatment |
| [133] |
| PBS/bamboo fiber | Hot press molding | Acetoxylation |
| [134] |
| PBS/Natural Fiber | Preparation Method | Optimum Concentration (%) of the Fiber | Type of Modifier | Mechanical Properties | Refs |
|---|---|---|---|---|---|
| PBS/Palm fiber (PF) and glycidyl methacrylate-grafted poly(butylene succinate) (PBS-g-GMA)/PF | Melt mixing at 140–150 °C | 40 wt.% for modified fiber composites. | Glycidyl methacrylate-grafting |
| [136] |
| PBS/Ramie fibers | Twin-screw extrusion and injection molding | 30% of the ramie content. | Single ramie fiber was modified with silane, alkali, acetic anhydride, and maleic anhydride treatment. |
| [137] |
| PBS/rice straw fiber | Injection molding | 30% of silane treated fibers | 3-aminopropyltriethoxysilane (APTES), 3-aminopropyltrimethoxysilane (APTMES), 3-(2-aminoethylaminopropyl)-triethoxysilane (AEAPTES) and 3-(2-aminoethylaminopropyl)-trimethoxysilane (AEAPTMES) fiber treatment. |
| [128] |
| PBS/curaua fiber | Thermo-pressing molding | 30 wt.% of the curaua fiber. | Alcohol and cyclohexane fiber pre-treatment. |
| [138] |
| PBS/oil palm mesocarp fiber (OPMF) and PBS/oil palm empty fruit bunch fiber (OPEFBF) | Melt blending | Various contents of the fiber resulted in improvement of various parameters of mechanical properties. | Fibers were washed with distilled water and acetone. |
| [139] |
| PBS/Natural Fiber or PBS Blend Natural Fiber Composites | Preparation Method | Intended Application | Summary of Results | Refs |
|---|---|---|---|---|
| Poly(butylene succinate) (PBS)/cellulose nanocrystals (CNC) bio-composite scaffolds | Electrospinning technique | Tissue engineering |
| [147] |
| Poly(butylene succinate)/date palm fibers (DPF) biocomposites | Injection molding process | Green and sustainable products |
| [148] |
| Polybutylene-succinate-modified Tapioca starch blend/Empty fruit brunch (EFB) composite films | Hot press technique | Agricultural munch films |
| [149] |
| Poly(lactic acid)/poly(butylene succinate)/cellulose fiber composite foams | Twin screw extrusion | Hot cups packaging |
| [150] |
| Poly(butylene succinate)-Poly(lactic acid) blend/wood flour | Hot melt blending and hot pressing | Used in diverse commercial applications |
| [151] |
| Poly(butylene succinate)/hemp fiber composites | Film stacking compression molding method followed by extrusion and injection molding | Low cost composite materials |
| [152] |
| Lignin/poly(butylene succinate) composites | Hot melt extrusion | Biomedical applications |
| [153] |
| Sustainable tetra pak recycled cellulose (rCell)/poly(butylene succinate) woody-like composites | Melt compounding using a Brabender mixer | Building materials, furniture and food packaging |
| [154] |
| Poly(butylene succinate)-isora nanofibil (PBS-INF) composites | Melt mixing of PBS with different loadings of INF | Automotive interior and food packaging | The incorporation of INF had a positive influence on the thermo-physical properties of PBS. | [155] |
| Poly(butylene succinate)/microcrystalline cellulose (MCC)/nanofibrillated cellulose (NFC) sustainable polymer composites | Melt mixing in a Brabender at 140 °C and rotation speed of 70 rpm | Packaging, medicine, automotive, construction, sustainable housing |
| [156] |
| Poly(butylene succinate)/cellulose nanocrystals (PBS/CNCs) composite scaffolds. | Supercritical carbon dioxide (Sc-CO2) foaming process | Tissue engineering |
| [157] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mochane, M.J.; Magagula, S.I.; Sefadi, J.S.; Mokhena, T.C. A Review on Green Composites Based on Natural Fiber-Reinforced Polybutylene Succinate (PBS). Polymers 2021, 13, 1200. https://doi.org/10.3390/polym13081200
Mochane MJ, Magagula SI, Sefadi JS, Mokhena TC. A Review on Green Composites Based on Natural Fiber-Reinforced Polybutylene Succinate (PBS). Polymers. 2021; 13(8):1200. https://doi.org/10.3390/polym13081200
Chicago/Turabian StyleMochane, Mokgaotsa J., Sifiso I. Magagula, Jeremia S. Sefadi, and Teboho C. Mokhena. 2021. "A Review on Green Composites Based on Natural Fiber-Reinforced Polybutylene Succinate (PBS)" Polymers 13, no. 8: 1200. https://doi.org/10.3390/polym13081200