Waste-to-Fuels: Pyrolysis of Low-Density Polyethylene Waste in the Presence of H-ZSM-11
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Catalyst Characterization
2.3. Pyrolysis Experimental Setup
2.4. Product Analysis
3. Results and Discussion
3.1. Characterization of Feedstock and Catalyst
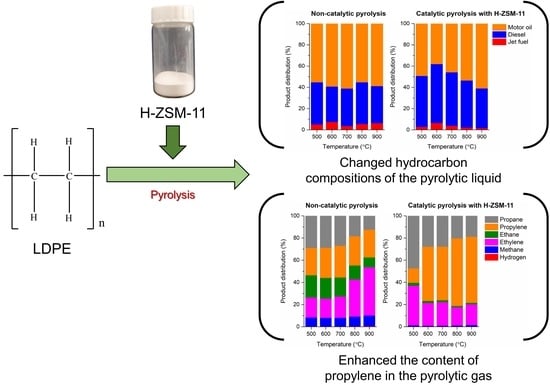
3.2. Pyrolysis of LDPE without and with H-ZSM-11 Catalyst
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Williams, P.T. Waste Treatment and Disposal, 2nd ed.; John Wiley & Sons: Chichester, UK, 2005. [Google Scholar]
- Tajima, S.; Komvopoulos, K. Surface modification of low-density polyethylene by inductively coupled argon plasma. J. Phys. Chem. B 2005, 109, 17623–17629. [Google Scholar] [CrossRef]
- Caldwell, R.A.; Woodell, J.E.; Ho, S.P.; Shalaby, S.W.; Boland, T.; Langan, E.M.; Laberge, M. In vitro evaluation of phosphonylated low-density polyethylene for vascular applications. J. Biomed. Mater. Res. 2002, 62, 514–524. [Google Scholar] [CrossRef]
- Ochoa, R.; Van Woert, H.; Lee, W.H.; Subramanian, R.; Kugler, E.; Eklund, P.C. Catalytic degradation of medium density polyethylene over silica-alumina supports. Fuel Process. Technol. 1996, 49, 119–136. [Google Scholar] [CrossRef]
- Ding, W.B.; Tuntawiroon, W.; Liang, J.; Anderson, L.L. Depolymerization of waste plastics with coal over metal-loaded silica-alumina catalysts. Fuel Process. Technol. 1996, 49, 49–63. [Google Scholar] [CrossRef]
- Ballice, L. Classification of volatile products evolved during temperature-programmed co-pyrolysis of low-density polyethylene (LDPE) with polypropylene (PP). Fuel 2002, 81, 1233–1240. [Google Scholar] [CrossRef]
- Shah, N.; Rockwell, J.; Huffman, G.P. Conversion of waste plastic to oil: direct liquefaction versus pyrolysis and hydroprocessing. Energy Fuels 1999, 13, 832–838. [Google Scholar] [CrossRef]
- Huffman, G.P.; Shah, N.; Shelley, M.; El-Halwagi, M.; Schindler, H.; Eastman, M. Feasibility study for a demonstration plant for liquefaction and coprocessing of waste plastics and tires. In Proceedings of the 23rd International Technical Conference on Coal Utilization and Fuel Systems, Clearwater, FL, USA, 9–13 March 1998. [Google Scholar]
- Buekens, A. Introduction to Feedstock Recycling of Plastics. In Feedstock Recycling and Pyrolysis of Waste Plastics; Scheirs, J., Kaminsky, W., Eds.; John Wiley & Sons: New York, NY, USA, 2006. [Google Scholar]
- Chenier, P.J. Survey of Industrial Chemistry, 3rd ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Zaman, A.U. Life cycle environmental assessment of municipal solid waste to energy technologies. Glob. J. Environ. Res. 2009, 3, 155–163. [Google Scholar]
- Mordi, R.C.; Fields, R.; Dwyer, J. Thermolysis of low density polyethylene catalysed by zeolites. J. Anal. Appl. Pyrolysis 1994, 29, 45–55. [Google Scholar] [CrossRef]
- Williams, P.T.; Williams, E.A. Fluidised bed pyrolysis of low density polyethylene to produce petrochemical feedstock. J. Anal. Appl. Pyrolysis 1999, 51, 107–126. [Google Scholar] [CrossRef]
- Marcilla, A.; Gómez-Siurana, A.; Valdés, F. Catalytic pyrolysis of LDPE over H-beta and HZSM-5 zeolites in dynamic conditions: Study of the evolution of the process. J. Anal. Appl. Pyrolysis 2007, 79, 433–442. [Google Scholar] [CrossRef]
- Kokotailo, G.T.; Chu, P.; Lawton, S.L.; Meier, W.M. Synthesis and structure of synthetic zeolite ZSM-11. Nature 1978, 275, 119–120. [Google Scholar] [CrossRef]
- Bibby, D.M.; Milestone, N.B.; Aldridge, L.P. Silicalite-2, a silica analogue of the aluminosilicate zeolite ZSM-11. Nature 1979, 280, 664–665. [Google Scholar] [CrossRef]
- Fyfe, C.A.; Gies, H.; Kokotailo, G.T.; Pasztor, C.; Strobl, H.; Cox, D.E. Detailed investigation of the lattice structure of zeolite ZSM-11 by a combination of solid-state NMR and synchrotron x-ray diffraction techniques. J. Am. Chem. Soc. 1989, 111, 2470–2474. [Google Scholar] [CrossRef]
- Wen, Z.; Yang, D.; He, X.; Li, Y.; Zhu, X. Methylation of benzene with methanol over HZSM-11 and HZSM-5: A density functional theory study. J. Mol. Catal. A Chem. 2016, 424, 351–357. [Google Scholar] [CrossRef]
- Bortnovsky, O.; Sazama, P.; Wichterlova, B. Cracking of pentenes to C2–C4 light olefins over zeolites and zeotypes: Role of topology and acid site strength and concentration. Appl. Catal. A Gen. 2005, 287, 203–213. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, H.; Li, X.; Xie, S.; Wang, Y.; Xin, W.; Liu, S.; Xu, L. Differences between ZSM-5 and ZSM-11 zeolite catalysts in 1-hexene aromatization and isomerization. Fuel Process. Technol. 2010, 91, 449–455. [Google Scholar] [CrossRef]
- Bleken, F.; Skistad, W.; Barbera, K.; Kustova, M.; Bordiga, S.; Beato, P.; Lillerud, K.P.; Svelle, S.; Olsbye, U. Conversion of methanol over 10-ring zeolites with differing volumes at channel intersections: Comparison of TNU-9, IM-5, ZSM-11 and ZSM-5. Phys. Chem. Chem. Phys. 2011, 13, 2539–2549. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.-M.; Oh, J.-I.; Baek, K.; Lee, J.; Kwon, E.E. Biodiesel production from waste cooking oil using biochar derived from chicken manure as a porous media and catalyst. Energy Convers. Manag. 2018, 165, 628–633. [Google Scholar] [CrossRef]
- Kwon, E.E.; Lee, T.; Ok, Y.S.; Tsang, D.C.W.; Park, C.; Lee, J. Effects of calcium carbonate on pyrolysis of sewage sludge. Energy 2018, 153, 726–731. [Google Scholar] [CrossRef]
- Kim, S.; Tsang, Y.F.; Kwon, E.E.; Lin, K.-Y.A.; Lee, J. Recently developed methods to enhance stability of heterogeneous catalysts for conversion of biomass-derived feedstocks. Korean J. Chem. Eng. 2019, 36, 1–11. [Google Scholar] [CrossRef]
- Kim, S.; Kwon, E.E.; Kim, Y.T.; Jung, S.; Kim, H.J.; Huber, G.W.; Lee, J. Recent advances in hydrodeoxygenation of biomass-derived oxygenates over heterogeneous catalysts. Green Chem. 2019, 21, 3715–3743. [Google Scholar] [CrossRef]
- Rodríguez-González, L.; Hermes, F.; Bertmer, M.; Rodríguez-Castellón, E.; Jiménez-López, A.; Simon, U. The acid properties of H-ZSM-5 as studied by NH3-TPD and 27Al-MAS-NMR spectroscopy. Appl. Catal. A Gen. 2007, 328, 174–182. [Google Scholar] [CrossRef]
- Luna-Murillo, B.; Pala, M.; Paioni, A.L.; Baldus, M.; Ronsse, F.; Prins, W.; Bruijnincx, P.C.A.; Weckhuysen, B.M. Catalytic fast pyrolysis of biomass: Catalyst characterization reveals the feed-dependent deactivation of a technical ZSM-5-based catalyst. ACS Sustain. Chem. Eng. 2021, 9, 291–304. [Google Scholar] [CrossRef]
- Nasir Uddin, M.; Daud, W.M.A.W.; Abbas, H.F. Potential hydrogen and non-condensable gases production from biomass pyrolysis: Insights into the process variables. Renew. Sustain. Energ. Rev. 2013, 27, 204–224. [Google Scholar] [CrossRef]
- Hossain, M.A.; Jewaratnam, J.; Ganesan, P.; Sahu, J.N.; Ramesh, S.; Poh, S.C. Microwave pyrolysis of oil palm fiber (OPF) for hydrogen production: Parametric investigation. Energy Convers. Manag. 2016, 115, 232–243. [Google Scholar] [CrossRef]
- Dixon, R.E. Conversion of Propylene into Ethylene. U.S. Patent US3485890A, 23 December 1969. [Google Scholar]
- Cho, S.-H.; Lee, S.S.; Jung, S.; Park, Y.-K.; Lin, K.-Y.A.; Lee, J.; Kwon, E.E. Carbon dioxide-cofeeding pyrolysis of pine sawdust over nickle-based catalyst for hydrogen production. Energy Convers. Manag. 2019, 201, 112140. [Google Scholar] [CrossRef]
- Shen, D.K.; Gu, S. The mechanism for thermal decomposition of cellulose and its main products. Bioresour. Technol. 2009, 100, 6496–6504. [Google Scholar] [CrossRef]
- Narbeshuber, T.F.; Brait, A.; Seshan, K.; Lercher, J.A. Dehydrogenation of light alkanes over zeolites. J. Catal. 1997, 172, 127–136. [Google Scholar] [CrossRef] [Green Version]
- Collins, C.D. Implementing phytoremediation of petroleum hydrocarbons. In Phytoremediation: Methods and Reviews; Willey, N., Ed.; Humana Press: Totowa, NJ, USA, 2007; pp. 99–108. [Google Scholar] [CrossRef]
- Czajczyńska, D.; Anguilano, L.; Ghazal, H.; Krzyżyńska, R.; Reynolds, A.J.; Spencer, N.; Jouhara, H. Potential of pyrolysis processes in the waste management sector. Therm. Sci. Eng. Prog. 2017, 3, 171–197. [Google Scholar] [CrossRef]




| Density (g mL−1) | Transition Temperature (°C) | Melting Point (°C) | Melt Index (g min−1) |
|---|---|---|---|
| 0.9 | 90 | 120 | 2.5 |
| Non-Catalytic Pyrolysis | Catalytic Pyrolysis with H-ZSM-11 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | 500 | 600 | 700 | 800 | 900 | 500 | 600 | 700 | 800 | 900 |
| C8 | 0 | 0 | 0 | 0 | 0 | 0 | 0.11 | 0.09 | 0.04 | 0.15 |
| C9 | 0 | 0 | 0 | 0 | 0 | 0 | 0.07 | 0 | 0 | 0 |
| C10 | 0.03 | 0.14 | 0.07 | 0.03 | 0.15 | 0.10 | 0.34 | 0.16 | 0.00 | 0 |
| C11 | 0.19 | 0.38 | 0.15 | 0.24 | 0.40 | 0.12 | 0.39 | 0.23 | 0.12 | 0 |
| C12 | 0.54 | 0.94 | 0.38 | 0.59 | 0.81 | 0.40 | 0.97 | 0.34 | 0.26 | 0.08 |
| C13 | 1.32 | 2.05 | 0.88 | 1.40 | 1.73 | 0.61 | 1.69 | 1.04 | 0.50 | 0.27 |
| C14 | 2.96 | 3.88 | 2.02 | 3.11 | 3.12 | 1.53 | 2.79 | 1.88 | 1.16 | 1.00 |
| C15 | 4.66 | 5.25 | 3.11 | 4.80 | 4.43 | 2.84 | 4.98 | 3.96 | 2.31 | 1.99 |
| C16 | 6.59 | 6.73 | 4.94 | 6.53 | 5.84 | 4.99 | 6.53 | 5.71 | 4.04 | 3.11 |
| C17 | 7.36 | 7.14 | 6.29 | 7.01 | 6.18 | 5.29 | 9.81 | 7.23 | 7.35 | 5.17 |
| C18 | 0.08 | 0.30 | 0.18 | 0.20 | 0.15 | 8.99 | 9.44 | 9.73 | 7.96 | 7.45 |
| C19 | 14.86 | 13.73 | 14.26 | 14.41 | 12.22 | 12.22 | 10.81 | 9.37 | 8.21 | 7.59 |
| C20 | 0.03 | 0.14 | 0.16 | 0.15 | 0.12 | 7.80 | 8.32 | 8.62 | 7.87 | 6.17 |
| C21 | 6.11 | 0.00 | 6.38 | 6.23 | 6.04 | 5.76 | 5.65 | 5.70 | 6.56 | 5.96 |
| C22 | 0 | 0 | 0 | 0 | 0 | 0.24 | 0.24 | 0.05 | 0 | 0 |
| C23 | 0.09 | 0.03 | 0.11 | 0.22 | 0.21 | 3.09 | 1.44 | 2.78 | 2.22 | 1.33 |
| C24 | 6.70 | 6.47 | 7.06 | 6.99 | 6.18 | 0 | 0 | 0 | 0 | 0 |
| C26 | 28.37 | 32.84 | 28.44 | 29.03 | 32.69 | 1.32 | 0.63 | 0.91 | 0.30 | 0.18 |
| C27 | 0 | 0 | 3.93 | 0 | 0 | 5.93 | 5.32 | 5.76 | 6.06 | 4.40 |
| C28 | 4.62 | 2.54 | 2.60 | 4.75 | 1.59 | 4.61 | 4.61 | 4.78 | 2.52 | 5.71 |
| C29 | 1.03 | 2.01 | 2.69 | 1.23 | 4.97 | 0 | 4.32 | 0 | 0 | 0 |
| C35 | 11.74 | 12.03 | 14.32 | 10.71 | 11.81 | 34.16 | 21.54 | 31.68 | 42.51 | 49.43 |
| C43 | 0.88 | 1.19 | 0 | 0 | 1.38 | 0 | 0 | 0 | 0 | 0 |
| C44 | 1.85 | 2.22 | 2.04 | 2.38 | 0 | 0 | 0 | 0 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, N.; Joo, J.; Lin, K.-Y.A.; Lee, J. Waste-to-Fuels: Pyrolysis of Low-Density Polyethylene Waste in the Presence of H-ZSM-11. Polymers 2021, 13, 1198. https://doi.org/10.3390/polym13081198
Lee N, Joo J, Lin K-YA, Lee J. Waste-to-Fuels: Pyrolysis of Low-Density Polyethylene Waste in the Presence of H-ZSM-11. Polymers. 2021; 13(8):1198. https://doi.org/10.3390/polym13081198
Chicago/Turabian StyleLee, Nahyeon, Junghee Joo, Kun-Yi Andrew Lin, and Jechan Lee. 2021. "Waste-to-Fuels: Pyrolysis of Low-Density Polyethylene Waste in the Presence of H-ZSM-11" Polymers 13, no. 8: 1198. https://doi.org/10.3390/polym13081198