Cosolvent-Driven Interfacial Polymerization for Superior Separation Performance of Polyurea-Based Pervaporation Membrane
Abstract
:1. Introduction
2. Methods
2.1. Experimental Chemicals
2.2. Preparation of Substrate
2.3. Preparation of Composite Membrane
2.4. Evaluation of Membrane
2.4.1. Field Emission Scanning Electron Microscopy
2.4.2. Attenuated Total Reflection Fourier Transform Infrared (ATR-FTIR) Spectrometry
2.4.3. Contact Angle Measurement
2.4.4. Pervaporation Test
3. Results and Discussion
3.1. Characterization of Membrane
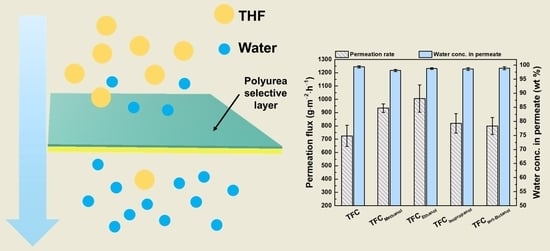
3.2. Identification of Best Cosolvent
3.3. Optimum Conditions for Composite Membrane Manufactured via the Best Cosolvent
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Raaijmakers, M.J.T.; Benes, N.E. Current trends in interfacial polymerization chemistry. Prog. Polym. Sci. 2016, 63, 86–142. [Google Scholar] [CrossRef]
- Gohil, J.M.; Ray, P. A review on semi-aromatic polyamide tfc membranes prepared by interfacial polymerization: Potential for water treatment and desalination. Sep. Purif. Technol. 2017, 181, 159–182. [Google Scholar] [CrossRef]
- Lee, K.P.; Arnot, T.C.; Mattia, D. A review of reverse osmosis membrane materials for desalination—development to date and future potential. J. Membr. Sci. 2011, 370, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.J.; Choi, K.; Baek, Y.; Kim, D.G.; Shim, J.; Yoon, J.; Lee, J.C. High-performance reverse osmosis cnt/polyamide nanocomposite membrane by controlled interfacial interactions. ACS Appl. Mater. Interfaces 2014, 6, 2819–2829. [Google Scholar] [CrossRef] [PubMed]
- Ang, M.B.M.Y.; Ji, Y.L.; Huang, S.H.; Lee, K.R.; Lai, J.Y. A facile and versatile strategy for fabricating thin-film nanocomposite membranes with polydopamine-piperazine nanoparticles generated in situ. J. Membr. Sci. 2019, 579, 79–89. [Google Scholar] [CrossRef]
- Ang, M.B.M.Y.; Tang, C.L.; De Guzman, M.R.; Maganto, H.L.C.; Caparanga, A.R.; Huang, S.H.; Tsai, H.A.; Hu, C.C.; Lee, K.R.; Lai, J.Y. Improved performance of thin-film nanofiltration membranes fabricated with the intervention of surfactants having different structures for water treatment. Desalination 2020, 481, 114352. [Google Scholar] [CrossRef]
- Bagheripour, E.; Moghadassi, A.; Hosseini, S.; Ray, M.; Parvizian, F.; Van der Bruggen, B. Highly hydrophilic and antifouling nanofiltration membrane incorporated with water-dispersible composite activated carbon/chitosan nanoparticles. Chem. Eng. Res. Des. 2018, 132, 812–821. [Google Scholar] [CrossRef]
- De Guzman, M.R.; Ang, M.B.M.Y.; Yeh, Y.-L.; Yang, H.-L.; Huang, S.-H.; Lee, K.-R. Improved pervaporation efficiency of thin-film composite polyamide membranes fabricated through acetone-assisted interfacial polymerization. Chem. Eng. Res. Des. 2021, 165, 375–385. [Google Scholar] [CrossRef]
- Li, P.; Shen, K.; Zhang, T.; Ding, S.; Wang, X. High-performance polyamide composite membranes via double-interfacial polymerizations on a nanofibrous substrate for pervaporation dehydration. Sep. Purif. Technol. 2021, 257, 117927. [Google Scholar] [CrossRef]
- Wang, C.; Li, Z.; Chen, J.; Li, Z.; Yin, Y.; Cao, L.; Zhong, Y.; Wu, H. Covalent organic framework modified polyamide nanofiltration membrane with enhanced performance for desalination. J. Membr. Sci. 2017, 523, 273–281. [Google Scholar] [CrossRef]
- Ang, M.B.M.Y.; Huang, S.H.; Li, Y.C.; Cahatol, A.T.C.; Tayo, L.L.; Hung, W.S.; Tsai, H.A.; Hu, C.C.; Lee, K.R.; Lai, J.Y. High-performance thin-film composite polyetheramide membranes for the dehydration of tetrahydrofuran. J. Membr. Sci. 2020, 611, 118373. [Google Scholar] [CrossRef]
- An, Q.F.; Ang, M.B.M.Y.; Huang, Y.H.; Huang, S.H.; Chia, Y.H.; Lai, C.L.; Tsai, H.A.; Hun, W.S.; Hu, C.C.; Wu, Y.P.; et al. Microstructural characterization and evaluation of pervaporation performance of thin-film composite membranes fabricated through interfacial polymerization on hydrolyzed polyacrylonitrile substrate. J. Membr. Sci. 2019, 583, 31–39. [Google Scholar] [CrossRef]
- Cheng, J.; Shi, W.; Zhang, L.; Zhang, R. A novel polyester composite nanofiltration membrane formed by interfacial polymerization of pentaerythritol (pe) and trimesoyl chloride (tmc). Appl. Surf. Sci. 2017, 416, 152–159. [Google Scholar] [CrossRef]
- Kim, B.-H.; Lee, C.-W.; Gong, M.-S. Preparation of polyesteramides based on aliphatic amine-containing phenol derivatives via interfacial polymerization. Macromol. Res. 2003, 11, 328–333. [Google Scholar] [CrossRef]
- Mah, K.H.; Yussof, H.W.; Abu Seman, M.N.; Mohammad, A.W. Optimisation of interfacial polymerization factors in thin-film composite (tfc) polyester nanofiltration (nf) membrane for separation of xylose from glucose. Sep. Purif. Technol. 2019, 209, 211–222. [Google Scholar] [CrossRef]
- Tang, B.; Huo, Z.; Wu, P. Study on a novel polyester composite nanofiltration membrane by interfacial polymerization of triethanolamine (teoa) and trimesoyl chloride (tmc). J. Membr. Sci. 2008, 320, 198–205. [Google Scholar] [CrossRef]
- Liu, X.-W.; Cao, Y.; Li, Y.-X.; Xu, Z.-L.; Li, Z.; Wang, M.; Ma, X.-H. High-performance polyamide/ceramic hollow fiber tfc membranes with tio2 interlayer for pervaporation dehydration of isopropanol solution. J. Membr. Sci. 2019, 576, 26–35. [Google Scholar] [CrossRef]
- Nazia, S.; Sekhar, S.C.; Jegatheesan, V.; Bhargava, S.K.; Sridhar, S. Performance of chemically resistant polyurea reverse osmosis membrane in the treatment of highly alkaline industrial wastewater containing sodium aluminate. Water Sci. Technol. 2020, 82, 2259–2270. [Google Scholar] [CrossRef]
- Rozelle, L.; Cadotte, J.; Cobian, K.; Kopp, C., Jr. Nonpolysaccharide membranes for reverse osmosis: Ns-100 membranes. In Reverse Osmosis and Synthetic Membranes; National Research Council of Canada Ottawa: Ottawa, ON, Canada, 1977; pp. 249–261. [Google Scholar]
- Zhao, C.-T.; Norberta de Pinho, M. Design of polypropylene oxide/polybutadiene bi-soft segment urethane/urea polymer for pervaporation membranes. Polymer 1999, 40, 6089–6097. [Google Scholar] [CrossRef]
- Das, S.; Banthia, A.K.; Adhikari, B. Pervaporation separation of dmf from water using a crosslinked polyurethane urea-pmma ipn membrane. Desalination 2006, 197, 106–116. [Google Scholar] [CrossRef]
- Liu, L.; Yu, S.; Zhou, Y.; Gao, C. Study on a novel polyamide-urea reverse osmosis composite membrane (icic–mpd)i. Preparation and characterization of icic–mpd membrane. J. Membr. Sci. 2006, 281, 88–94. [Google Scholar] [CrossRef]
- Das, S.; Banthia, A.K.; Adhikari, B. Porous polyurethane urea membranes for pervaporation separation of phenol and chlorophenols from water. Chem. Eng. J. 2008, 138, 215–223. [Google Scholar] [CrossRef]
- Sadeghi, M.; Semsarzadeh, M.A.; Barikani, M.; Ghalei, B. The effect of urethane and urea content on the gas permeation properties of poly(urethane-urea) membranes. J. Membr. Sci. 2010, 354, 40–47. [Google Scholar] [CrossRef]
- Koczka, K.; Manczinger, J.; Mizsey, P.; Fonyo, Z. Novel hybrid separation processes based on pervaporation for thf recovery. Chem. Eng. Process. 2007, 46, 239–246. [Google Scholar] [CrossRef]
- Li, S.; Tuan, V.A.; Noble, R.D.; Falconer, J.L. Pervaporation of water/thf mixtures using zeolite membranes. Ind. Eng. Chem. Res. 2001, 40, 4577–4585. [Google Scholar] [CrossRef]
- Slater, C.S.; Savelski, M.J.; Moroz, T.M.; Raymond, M.J. Pervaporation as a green drying process for tetrahydrofuran recovery in pharmaceutical synthesis. Green Chem. Lett. Rev. 2012, 5, 55–64. [Google Scholar] [CrossRef]
- Marquez, J.A.D.; Ang, M.B.M.Y.; Doma, B.T.; Huang, S.H.; Tsai, H.A.; Lee, K.R.; Lai, J.Y. Application of cosolvent-assisted interfacial polymerization technique to fabricate thin-film composite polyamide pervaporation membranes with pvdf hollow fiber as support. J. Membr. Sci. 2018, 564, 722–731. [Google Scholar] [CrossRef]
- Lee, J.; Wang, R.; Bae, T.-H. A comprehensive understanding of co-solvent effects on interfacial polymerization: Interaction with trimesoyl chloride. J. Membr. Sci. 2019, 583, 70–80. [Google Scholar] [CrossRef]
- Kong, C.L.; Shintani, T.; Kamada, T.; Freger, V.; Tsuru, T. Co-solvent-mediated synthesis of thin polyamide membranes. J. Membr. Sci. 2011, 384, 10–16. [Google Scholar] [CrossRef]
- Esfandian, F.; Peyravi, M.; Ghoreyshi, A.A.; Jahanshahi, M.; Rad, A.S. Fabrication of tfc nanofiltration membranes via co-solvent assisted interfacial polymerization for lactose recovery. Arab. J. Chem. 2019, 12, 5325–5338. [Google Scholar] [CrossRef]
- De Guzman, M.R.; Ang, M.B.M.Y.; Huang, S.-H.; Jhuang, W.-R.; Tsai, H.-A.; Hu, C.-C.; Lee, K.-R.; Lai, J.-Y. Layer-by-layer self-assembly of polyethyleneimine and poly(4-styrene sulfonic acid-co-maleic acid) forming composite polyelectrolyte membranes for pervaporation of aqueous alcohol solutions. J. Polym. Res. 2019, 26, 286. [Google Scholar] [CrossRef]
- Hung, W.-S.; Ho, S.-Y.; Chiao, Y.-H.; Chan, C.-C.; Woon, W.-Y.; Yin, M.-J.; Chang, C.-Y.; Lee, Y.M.; An, Q.-F. Electrical tunable pvdf/graphene membrane for controlled molecule separation. Chem. Mater. 2020, 32, 5750–5758. [Google Scholar] [CrossRef]
- Maia, F.; Tedim, J.; Bastos, A.C.; Ferreira, M.G.S.; Zheludkevich, M.L. Active sensing coating for early detection of corrosion processes. RSC Adv. 2014, 4, 17780–17786. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Zheng, Z.; Chen, L.; Tu, X.; Wang, X. Glutathione-responsive biodegradable poly(urea-urethane)s containing l-cystine-based chain extender. J. Biomater. Sci. Polym. Ed. 2013, 24, 831–848. [Google Scholar] [CrossRef] [PubMed]
- Shalmashi, A.; Amani, F. Densities and excess molar volumes for binary solution of water+ ethanol,+ methanol and+ propanol from (283.15 to 313.15) k. Lat. Am. Appl. Res. 2014, 44, 163–166. [Google Scholar]
- Egorov, G.I.; Makarov, D.M. Densities and volume properties of (water+tert-butanol) over the temperature range of (274.15 to 348.15)k at pressure of 0.1mpa. J. Chem. Thermodyn. 2011, 43, 430–441. [Google Scholar] [CrossRef]
- Liu, M.; Zheng, Y.; Shuai, S.; Zhou, Q.; Yu, S.; Gao, C. Thin-film composite membrane formed by interfacial polymerization of polyvinylamine (pvam) and trimesoyl chloride (tmc) for nanofiltration. Desalination 2012, 288, 98–107. [Google Scholar] [CrossRef]
- Ni, H.; Nash, H.A.; Worden, J.G.; Soucek, M.D. Effect of catalysts on the reaction of an aliphatic isocyanate and water. J. Polym. Sci. Part A Polym. Chem. 2002, 40, 1677–1688. [Google Scholar] [CrossRef]
- Krone, C.A.; Klingner, T.D. Isocyanates, polyurethane and childhood asthma. Pediatr. Allergy Immunol. 2005, 16, 368–379. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Kondo, S.; Kikuchi, T.; Christiani, L.; Hwang, B.; Sasaki, K.; Nishihara, M. Development of a heat-treated polymer–polymer type charge-transfer blend membrane for application in polymer electrolyte fuel cells. ACS Appl. Energy Mater. 2019, 2, 8715–8723. [Google Scholar] [CrossRef]
- Hu, D.; Xu, Z.-L.; Wei, Y.-M. A high performance silica–fluoropolyamide nanofiltration membrane prepared by interfacial polymerization. Sep. Purif. Technol. 2013, 110, 31–38. [Google Scholar] [CrossRef]







| Membrane | Water Contact Angle (o) |
|---|---|
| TFC | 63.55 ± 2.07 |
| TFCMethanol | 61.40 ± 3.87 |
| TFCEthanol | 63.96 ± 5.18 |
| TFCIsopropanol | 66.31 ± 2.57 |
| TFCtert-Butanol | 67.40 ± 2.86 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Guzman, M.R.; Ang, M.B.M.Y.; Huang, S.-H.; Hu, F.-C.; Chiao, Y.-H.; Tsai, H.-A.; Lee, K.-R. Cosolvent-Driven Interfacial Polymerization for Superior Separation Performance of Polyurea-Based Pervaporation Membrane. Polymers 2021, 13, 1179. https://doi.org/10.3390/polym13081179
De Guzman MR, Ang MBMY, Huang S-H, Hu F-C, Chiao Y-H, Tsai H-A, Lee K-R. Cosolvent-Driven Interfacial Polymerization for Superior Separation Performance of Polyurea-Based Pervaporation Membrane. Polymers. 2021; 13(8):1179. https://doi.org/10.3390/polym13081179
Chicago/Turabian StyleDe Guzman, Manuel Reyes, Micah Belle Marie Yap Ang, Shu-Hsien Huang, Fang-Chi Hu, Yu-Hsuan Chiao, Hui-An Tsai, and Kueir-Rarn Lee. 2021. "Cosolvent-Driven Interfacial Polymerization for Superior Separation Performance of Polyurea-Based Pervaporation Membrane" Polymers 13, no. 8: 1179. https://doi.org/10.3390/polym13081179