Novel Graphene Oxide Nanohybrid Doped Methacrylic Acid Hydrogels for Enhanced Swelling Capability and Cationic Adsorbability
Abstract
:1. Introduction
2. Experiments
2.1. Materials
2.2. Methods
2.2.1. Synthesis of Graphene Oxide (GO)
2.2.2. Preparation of PMAA/GO Hydrogel
2.2.3. Structural Characterization
2.2.4. Swelling Rate Characteristic of PMAA/GO Hydrogel
2.2.5. Adsorption Performance of Hydrogels for Methylene Blue
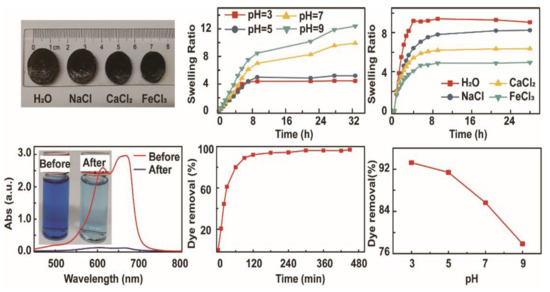
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, H.; Zhang, N.; Ma, M. Electroconductive hydrogels for biomedical applications. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2019, 11, e1568. [Google Scholar] [CrossRef]
- Palmese, L.L.; Thapa, R.K.; Sullivan, M.O.; Kiick, K.L. Hybrid hydrogels for biomedical applications. Curr. Opin. Chem. Eng. 2019, 24, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Greene, A.F.; Danielson, M.K.; Delawder, A.O.; Liles, K.P.; Li, X.; Natraj, A.; Wellen, A.; Barnes, J.C. Redox-Responsive Artificial Molecular Muscles: Reversible Radical-Based Self-Assembly for Actuating Hydrogels. Chem. Mater. 2017, 29, 9498–9508. [Google Scholar] [CrossRef]
- Duan, X.; Yu, J.; Zhu, Y.; Zheng, Z.; Liao, Q.; Xiao, Y.; Li, Y.; He, Z.; Zhao, Y.; Wang, H.; et al. Large-Scale Spinning Approach to Engineering Knittable Hydrogel Fiber for Soft Robots. ACS Nano 2020, 14, 14929–14938. [Google Scholar] [CrossRef]
- Yuk, H.; Lin, S.; Ma, C.; Takaffoli, M.; Fang, N.X.; Zhao, X. Hydraulic hydrogel actuators and robots optically and sonically camouflaged in water. Nat. Commun. 2017, 8, 14230. [Google Scholar] [CrossRef] [Green Version]
- Guidetti, G.; Giuri, D.; Zanna, N.; Calvaresi, M.; Montalti, M.; Tomasini, C. Biocompatible and Light-Penetrating Hydrogels for Water Decontamination. ACS Omega 2018, 3, 8122–8128. [Google Scholar] [CrossRef] [PubMed]
- Van Tran, V.; Park, D.; Lee, Y.-C. Hydrogel applications for adsorption of contaminants in water and wastewater treatment. Environ. Sci. Pollut. Res. 2018, 25, 24569–24599. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Servant, A.; Guy, O.J.; Al-Jamal, K.T.; Williams, P.R.; Hawkins, K.M.; Kostarelos, K. An electric-field responsive microsystem for controllable miniaturised drug delivery applications. Sens. Actuators B Chem. 2012, 175, 100–105. [Google Scholar] [CrossRef]
- Ying, X.; Wang, Y.; Liang, J.; Yue, J.; Xu, C.; Lu, L.; Xu, Z.; Gao, J.; Du, Y.; Chen, Z. Angiopep-Conjugated Electro-Responsive Hydrogel Nanoparticles: Therapeutic Potential for Epilepsy. Angew. Chem. Int. Ed. 2014, 53, 12436–12440. [Google Scholar] [CrossRef]
- Ganguly, S.; Das, P.; Maity, P.P.; Mondal, S.; Ghosh, S.; Dhara, S.; Das, N.C. Green Reduced Graphene Oxide Toughened Semi-IPN Monolith Hydrogel as Dual Responsive Drug Release System: Rheological, Physicomechanical, and Electrical Evaluations. J. Phys. Chem. B 2018, 122, 7201–7218. [Google Scholar] [CrossRef]
- Yan, J.; Zhu, J.; Cui, M.; Zhang, J.; Ma, F.; Su, Y.; Han, X. Multifunctional Mineral Hydrogels: Potential in Artificially Intelligent Skins and Drug Delivery. ACS Omega 2019, 4, 19145–19152. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, S.; Ray, D.; Das, P.; Maity, P.P.; Mondal, S.; Aswal, V.; Dhara, S.; Das, N.C. Mechanically robust dual responsive water dispersible-graphene based conductive elastomeric hydrogel for tunable pulsatile drug release. Ultrason. Sonochem. 2018, 42, 212–227. [Google Scholar] [CrossRef] [PubMed]
- Milosavljevic, N.B.; Ristic, M.D.; Peric-Grujic, A.A.; Filipovic, J.M.; Strbac, S.B.; Rakocevic, Z.; Kalagasidis Krusic, M.T. Sorption of zinc by novel pH-sensitive hydrogels based on chitosan, itaconic acid and methacrylic acid. J. Hazard. Mater. 2011, 192, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-D.; Ren, P.-G.; Chen, J.; Zhang, W.-Q.; Ji, X.; Li, Z.-M. High barrier graphene oxide nanosheet/poly(vinyl alcohol) nanocomposite films. J. Membr. Sci. 2012, 409-410, 156–163. [Google Scholar] [CrossRef]
- Martins, A.F.; Monteiro, J.P.; Bonafé, E.G.; Gerola, A.P.; Silva, C.T.; Girotto, E.M.; Rubira, A.F.; Muniz, E.C. Bactericidal activity of hydrogel beads based on N,N,N-trimethyl chitosan/alginate complexes loaded with silver nanoparticles. Chin. Chem. Lett. 2015, 26, 1129–1132. [Google Scholar] [CrossRef]
- Farhoudian, S.; Yadollahi, M.; Namazi, H. Facile synthesis of antibacterial chitosan/CuO bio-nanocomposite hydrogel beads. Int. J. Biol. Macromol. 2016, 82, 837–843. [Google Scholar] [CrossRef]
- Aminabhavi, T.; Dharupaneedi, S. Production of chitosan-based hydrogels for biomedical applications. Chitosan Based Biomater. 2017, 1, 295–319. [Google Scholar] [CrossRef]
- Holkar, C.R.; Jadhav, A.J.; Pinjari, D.V.; Mahamuni, N.M.; Pandit, A.B. A critical review on textile wastewater treatments: Possible approaches. J. Environ. Manag. 2016, 182, 351–366. [Google Scholar] [CrossRef]
- Tan, K.B.; Vakili, M.; Horri, B.A.; Poh, P.E.; Abdullah, A.Z.; Salamatinia, B. Adsorption of dyes by nanomaterials: Recent developments and adsorption mechanisms. Sep. Purif. Technol. 2015, 150, 229–242. [Google Scholar] [CrossRef]
- Yagub, M.T.; Sen, T.K.; Afroze, S.; Ang, H. Dye and its removal from aqueous solution by adsorption: A review. Adv. Colloid Interface Sci. 2014, 209, 172–184. [Google Scholar] [CrossRef]
- Pakdel, P.M.; Peighambardoust, S.J. A review on acrylic based hydrogels and their applications in wastewater treatment. J. Environ. Manag. 2018, 217, 123–143. [Google Scholar] [CrossRef] [PubMed]
- Lebeau, J.; Efromson, J.P.; Lynch, M.D. A Review of the Biotechnological Production of Methacrylic Acid. Front. Bioeng. Biotechnol. 2020, 8, 207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Wei, Y.; Ding, H.; Wu, Z.; Yang, X.; Li, Z.; Huang, W.; Xie, X.; Tao, K.; Wang, X. Green Synthesis of 3D Chemically Functionalized Graphene Hydrogel for High-Performance NH3 and NO2 Detection at Room Temperature. ACS Appl. Mater. Interfaces 2020, 12, 20623–20632. [Google Scholar] [CrossRef] [PubMed]
- Alammar, A.; Park, S.-H.; Ibrahim, I.; Arun, D.; Holtzl, T.; Dumée, L.F.; Lim, H.N.; Szekely, G. Architecting neonicotinoid-scavenging nanocomposite hydrogels for environmental remediation. Appl. Mater. Today 2020, 21, 100878. [Google Scholar] [CrossRef]
- Cao, J.; Wang, Y.; Wang, L.; Yu, F.; Ma, J. Na3V2(PO4)3@C as Faradaic Electrodes in Capacitive Deionization for High-Performance Desalination. Nano Lett. 2019, 19, 823–828. [Google Scholar] [CrossRef]
- Palmieri, V.; Papi, M.; Conti, C.; Ciasca, G.; Maulucci, G.; De Spirito, M. The future development of bacteria fighting medical devices: The role of graphene oxide. Expert Rev. Med. Devices 2016, 13, 1013–1019. [Google Scholar] [CrossRef]
- Tian, Y.; Yu, Z.; Cao, L.; Zhang, X.L.; Sun, C.; Wang, D.-W. Graphene oxide: An emerging electromaterial for energy storage and conversion. J. Energy Chem. 2021, 55, 323–344. [Google Scholar] [CrossRef]
- Gong, T.; Zhang, H.; Huang, W.; Mao, L.; Ke, Y.; Gao, M.; Yu, B. Highly responsive flexible strain sensor using polystyrene nanoparticle doped reduced graphene oxide for human health monitoring. Carbon 2018, 140, 286–295. [Google Scholar] [CrossRef]
- Ganesan, V.; Louis, C.; Damodaran, S.P. Graphene oxide-mesoporous iron oxide nanohybrid: An efficient reusable nanoadsorbent for the removal of organic dyes from wastewater. Mater. Res. Express 2019, 6, 850–858. [Google Scholar] [CrossRef]
- Xu, J.; Xu, D.; Zhu, B.; Cheng, B.; Jiang, C. Adsorptive removal of an anionic dye Congo red by flower-like hierarchical magnesium oxide (MgO)-graphene oxide composite microspheres. Appl. Surf. Sci. 2018, 435, 1136–1142. [Google Scholar] [CrossRef]
- Ruihong, X.; Penggang, R.; Jian, H.; Fang, R.; Lianzhen, R.; Zhenfeng, S. Preparation and properties of graphene oxide-regenerated cellulose/polyvinyl alcohol hydrogel with pH-sensitive behavior. Carbohydr. Polym. 2016, 138, 222–228. [Google Scholar] [CrossRef]
- Sarkar, S.D.; Uddin, M.; Roy, C.K.; Hossen, J.; Sujan, M.I.; Azam, S. Mechanically tough and highly stretchable poly(acrylic acid) hydrogel cross-linked by 2D graphene oxide. RSC Adv. 2020, 10, 10949–10958. [Google Scholar] [CrossRef] [Green Version]
- Ren, F.; Li, Z.; Tan, W.-Z.; Liu, X.-H.; Sun, Z.-F.; Ren, P.-G.; Yan, D.-X. Facile preparation of 3D regenerated cellulose/graphene oxide composite aerogel with high-efficiency adsorption towards methylene blue. J. Colloid Interface Sci. 2018, 532, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Alammar, A.; Park, S.-H.; Williams, C.J.; Derby, B.; Szekely, G. Oil-in-water separation with graphene-based nanocomposite membranes for produced water treatment. J. Membr. Sci. 2020, 603. [Google Scholar] [CrossRef]
- Zhang, Q.; Mao, Z.; Wang, K.; Phan, N.T.S.; Zhang, F. Microwave-assisted aqueous carbon–carbon cross-coupling reactions of aryl chlorides catalysed by reduced graphene oxide supported palladium nanoparticles. Green Chem. 2020, 22, 3239–3247. [Google Scholar] [CrossRef]
- Mikhaylov, A.A.; Medvedev, A.G.; Grishanov, D.A.; Edison, E.; Srinivasan, M.; Sladkevich, S.; Gun, J.; Prikhodchenko, P.V.; Lev, O. Green Synthesis of a Nanocrystalline Tin Disulfide-Reduced Graphene Oxide Anode from Ammonium Peroxostannate: A Highly Stable Sodium-Ion Battery Anode. ACS Sustain. Chem. Eng. 2020, 8, 5485–5494. [Google Scholar] [CrossRef]
- Chen, J.; Yao, B.; Li, C.; Shi, G. An improved Hummers method for eco-friendly synthesis of graphene oxide. Carbon 2013, 64, 225–229. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis ofGraphene Oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Mac Kenna, N.; Calvert, P.; Morrin, A.; Wallace, G.G.; Moulton, S.E. Electro-stimulated release from a reduced graphene oxide composite hydrogel. J. Mater. Chem. B 2015, 3, 2530–2537. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Cao, M.; Wang, C.; Ao, Y.; Hou, J.; Qian, J. Kinetics and thermodynamics of adsorption of methylene blue by a magnetic graphene-carbon nanotube composite. Appl. Surf. Sci. 2014, 290, 116–124. [Google Scholar] [CrossRef]
- Hui, W.; Wei, L.; Jiaojing, S.; Chen, Z.; Mingbo, W.; Baohua, L.; Quanhong, Y. pH-dependent size, surface chemistry and electrochemical properties of graphene oxide. New Carbon Mater. 2013, 28, 328–335. [Google Scholar] [CrossRef]
- Shi, H.; Li, W.; Zhong, L.; Xu, C. Methylene Blue Adsorption from Aqueous Solution by Magnetic Cellulose/Graphene Oxide Composite: Equilibrium, Kinetics, and Thermodynamics. Ind. Eng. Chem. Res. 2014, 53, 1108–1118. [Google Scholar] [CrossRef]
- Fan, S.; Wang, Y.; Wang, Z.; Tang, J.; Tang, J.; Li, X. Removal of methylene blue from aqueous solution by sewage sludge-derived biochar: Adsorption kinetics, equilibrium, thermodynamics and mechanism. J. Environ. Chem. Eng. 2017, 5, 601–611. [Google Scholar] [CrossRef]




| Kinetic Equations | Adsorption Kinetic Constant | R2 | |
|---|---|---|---|
| Quasi-first-order dynamics | Qe = 71.24 | k1 = 0.015 | 0.916 |
| Quasi-second-order dynamics | Qe = 126.42 | k2 = 4.10 × 10−4 | 0.989 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Lyu, Y.; Hu, Y.; An, J.; Chen, R.; Chen, M.; Du, J.; Hou, C. Novel Graphene Oxide Nanohybrid Doped Methacrylic Acid Hydrogels for Enhanced Swelling Capability and Cationic Adsorbability. Polymers 2021, 13, 1112. https://doi.org/10.3390/polym13071112
Liu Y, Lyu Y, Hu Y, An J, Chen R, Chen M, Du J, Hou C. Novel Graphene Oxide Nanohybrid Doped Methacrylic Acid Hydrogels for Enhanced Swelling Capability and Cationic Adsorbability. Polymers. 2021; 13(7):1112. https://doi.org/10.3390/polym13071112
Chicago/Turabian StyleLiu, Yufei, Ying Lyu, Yongqin Hu, Jia An, Rubing Chen, Meizhu Chen, Jihe Du, and Chen Hou. 2021. "Novel Graphene Oxide Nanohybrid Doped Methacrylic Acid Hydrogels for Enhanced Swelling Capability and Cationic Adsorbability" Polymers 13, no. 7: 1112. https://doi.org/10.3390/polym13071112