Development and In Vitro Evaluation of 2-Methoxyestradiol Loaded Polymeric Micelles for Enhancing Anticancer Activities in Prostate Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Formulation Development and Optimization of Polymeric Micelles
2.2.1. Design of Experiments
2.2.2. Formulation
2.2.3. Optimization of 2ME-PMs
2.3. Characterization of Drug-Loaded Polymeric Micelles
2.3.1. Particle Size, Polydispersity Index, and Zeta Potential of Polymeric Micelles
2.3.2. Surface Morphology of Optimized Polymeric Micelles Using TEM
2.3.3. Entrapment Efficiency
2.4. In Vitro Drug Release Study
2.5. In Vitro Cell Line Study in Prostate Cancer (PC-3 Cells)
2.5.1. Cell Viability Using MTT Assay
2.5.2. Apoptotic Activity by Flow Cytometry
2.5.3. Cell Cycle Analysis by Flow Cytometry
2.5.4. Mitochondrial Membrane Potential Activity
2.5.5. Estimation of Molecular Markers by ELISA Method
2.5.6. Effect of 2ME-PMs on Bax and Bcl-2 Using RT-PCR
2.6. Statistical Analysis
3. Results and Discussion
3.1. Formulation Development and Optimization of Polymeric Micelles
3.1.1. Design of Experiments
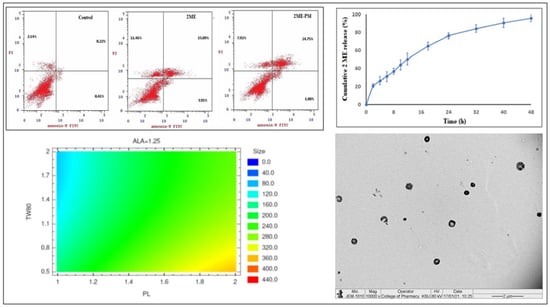
3.1.2. Optimization of 2ME-PMs
3.2. Characterization of Drug-Loaded Polymeric Micelles
3.2.1. Particle Size, Polydispersity Index, and Zeta Potential of Polymeric Micelles
3.2.2. Surface Morphology of Optimized Polymeric Micelles Using TEM
3.2.3. Entrapment Efficiency
3.3. In Vitro Drug Release Study
3.4. In Vitro Cell Line Study in Prostate Cancer (PC-3 Cells)
3.4.1. Cell Viability Using MTT Assay
3.4.2. Apoptotic Activity by Flow Cytometry
3.4.3. Cell Cycle Analysis by Flow Cytometry
3.4.4. Mitochondrial Membrane Potential Activity
3.4.5. Estimation of Molecular Markers by ELISA Method
3.4.6. Effect of 2ME-PMs on Bax and Bcl-2 Using RT-PCR
4. Practical Applications, Future Research Perspectives, and Challenges
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Sanna, V.; Sechi, M. Nanoparticle therapeutics for prostate cancer treatment. Maturitas 2012, 73, 27–32. [Google Scholar] [CrossRef]
- Vijjan, V.; Dubey, D. New therapeutic targets in the treatment of prostate cancer. Indian J. Urol. 2007, 23, 61–66. [Google Scholar]
- Aragon-Ching, J.B.; Madan, R.A. The path forward in prostate cancer therapeutics. Asian J. Androl. 2018, 20, 213–214. [Google Scholar] [CrossRef]
- Nelles, J.L.; Hu, W.-Y.; Prins, G.S. Estrogen action and prostate cancer. Expert Rev. Endocrinol. Metab. 2011, 6, 437–451. [Google Scholar] [CrossRef] [Green Version]
- Dubey, R.K.; Imthurn, B.; Jackson, E.K. 2-Methoxyestradiol: A Potential Treatment for Multiple Proliferative Disorders. Endocrinology 2007, 148, 4125–4127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sweeney, C.; Liu, G.; Yiannoutsos, C.; Kolesar, J.; Horvath, D.; Staab, M.J.; Fife, K.; Armstrong, V.; Treston, A.; Sidor, C.; et al. A phase II multicenter, randomized, double-blind, safety trial assessing the pharmacokinetics, pharmacodynamics, and efficacy of oral 2-methoxyestradiol capsules in hormone-refractory prostate cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2005, 11, 6625–6633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, X.; Chen, C.; Liu, X.; Hou, P.; Guo, X.; Ding, F.; Wang, Z.; Hu, Y.; Li, Z.; Zhang, Z. High oral bioavailability of 2-methoxyestradiol in PEG-PLGA micelles-microspheres for cancer therapy. Eur. J. Pharm. Biopharm. 2017, 117, 116–122. [Google Scholar] [CrossRef]
- Ireson, C.R.; Chander, S.K.; Purohit, A.; Perera, S.; Newman, S.P.; Parish, D.; Leese, M.P.; Smith, A.C.; Potter, B.V.L.; Reed, M.J. Pharmacokinetics and efficacy of 2-methoxyoestradiol and 2-methoxyoestradiol-bis-sulphamate in vivo in rodents. Br. J. Cancer 2004, 90, 932–937. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.-H.; Zhang, N.; Cui, F.-D.; Du, B.; Zhang, Z.-Z. An investigation on intestinal absorption of a new anticancer drug, 2-methoxyestradiol. Pharmazie 2009, 64, 748–751. [Google Scholar] [PubMed]
- Harrison, M.R.; Hahn, N.M.; Pili, R.; Oh, W.K.; Hammers, H.; Sweeney, C.; Kim, K.; Perlman, S.; Arnott, J.; Sidor, C.; et al. A phase II study of 2-methoxyestradiol (2ME2) NanoCrystal® dispersion (NCD) in patients with taxane-refractory, metastatic castrate-resistant prostate cancer (CRPC). Investig. New Drugs 2011, 29, 1465–1474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borahay, M.A.; Vincent, K.L.; Motamedi, M.; Tekedereli, I.; Salama, S.A.; Ozpolat, B.; Kilic, G.S. Liposomal 2-Methoxyestradiol Nanoparticles for Treatment of Uterine Leiomyoma in a Patient-Derived Xenograft Mouse Model. Reprod. Sci. 2021, 28, 271–277. [Google Scholar] [CrossRef]
- Pillai, G.J.; Paul-Prasanth, B.; Nair, S.V.; Menon, D. Influence of surface passivation of 2-Methoxyestradiol loaded PLGA nanoparticles on cellular interactions, pharmacokinetics and tumour accumulation. Colloids Surf. B Biointerfaces 2017, 150, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Alfaro, A.; León, A.; Guajardo-Correa, E.; Reúquen, P.; Torres, F.; Mery, M.; Segura, R.; Zapata, P.A.; Orihuela, P.A. MgO nanoparticles coated with polyethylene glycol as carrier for 2-Methoxyestradiol anticancer drug. PLoS ONE 2019, 14, e0214900. [Google Scholar] [CrossRef] [Green Version]
- Avramović, N.; Mandić, B.; Savić-Radojević, A.; Simić, T. Polymeric Nanocarriers of Drug Delivery Systems in Cancer Therapy. Pharmaceutics 2020, 12, 298. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Tang, X.; Zhang, J.; Lu, W.; Lin, X.; Zhang, Y.; Tian, B.; Yang, H.; He, H. PEG–PLGA copolymers: Their structure and structure-influenced drug delivery applications. J. Control. Release 2014, 183, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Li, K.; Wang, H. Polymeric micelles based on PEGylated chitosan-g-lipoic acid as carrier for efficient intracellular drug delivery. J. Biomater. Appl. 2016, 31, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- Alhakamy, N.A.; Fahmy, U.A.; Badr-Eldin, S.M.; Ahmed, O.A.A.; Asfour, H.Z.; Aldawsari, H.M.; Algandaby, M.M.; Eid, B.G.; Abdel-Naim, A.B.; Awan, Z.A.; et al. Optimized Icariin Phytosomes Exhibit Enhanced Cytotoxicity and Apoptosis-Inducing Activities in Ovarian Cancer Cells. Pharmaceutics 2020, 12, 346. [Google Scholar] [CrossRef]
- Zhang, N.; Xu, Y.; Xin, X.; Huo, P.; Zhang, Y.; Chen, H.; Feng, N.; Feng, Q.; Zhang, Z. Dual-modal imaging-guided theranostic nanocarriers based on 2-methoxyestradiol and indocyanine green. Int. J. Pharm. 2021, 592, 120098. [Google Scholar] [CrossRef]
- Shi, J.; Wang, Z.; Wang, L.; Wang, H.; Li, L.; Yu, X.; Zhang, J.; Ma, R.; Zhang, Z. Photodynamic therapy of a 2-methoxyestradiol tumor-targeting drug delivery system mediated by Asn-Gly-Arg in breast cancer. Int. J. Nanomed. 2013, 8, 1551–1562. [Google Scholar]
- Hsiao, K.Y.; Wu, Y.-J.; Liu, Z.N.; Chuang, C.W.; Huang, H.H.; Kuo, S.M. Anticancer Effects of Sinulariolide-Conjugated Hyaluronan Nanoparticles on Lung Adenocarcinoma Cells. Molecules 2016, 21, 297. [Google Scholar] [CrossRef] [Green Version]
- Xue, Y.; Chen, Q.; Ding, T.; Sun, J. SiO2 nanoparticle-induced impairment of mitochondrial energy metabolism in hepatocytes directly and through a Kupffer cell-mediated pathway in vitro. Int. J. Nanomed. 2014, 9, 2891–2903. [Google Scholar]
- Golestani Eimani, B.; Sanati, M.H.; Houshmand, M.; Ataei, M.; Akbarian, F.; Shakhssalim, N. Expression and prognostic significance of bcl-2 and bax in the progression and clinical outcome of transitional bladder cell carcinoma. Cell J. 2014, 15, 356–363. [Google Scholar]
- Bagheri, M.; Bresseleers, J.; Varela-Moreira, A.; Sandre, O.; Meeuwissen, S.A.; Schiffelers, R.M.; Metselaar, J.M.; van Nostrum, C.F.; van Hest, J.C.M.; Hennink, W.E. Effect of Formulation and Processing Parameters on the Size of mPEG- b-p(HPMA-Bz) Polymeric Micelles. Langmuir 2018, 34, 15495–15506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salimi, A.; Sharif Makhmal Zadeh, B.; Kazemi, M. Preparation and optimization of polymeric micelles as an oral drug delivery system for deferoxamine mesylate: In vitro and ex vivo studies. Res. Pharm. Sci. 2019, 14, 293–307. [Google Scholar]
- Chuacharoen, T.; Prasongsuk, S.; Sabliov, C.M. Effect of Surfactant Concentrations on Physicochemical Properties and Functionality of Curcumin Nanoemulsions Under Conditions Relevant to Commercial Utilization. Molecules 2019, 24, 2744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abourehab, M.A.; Ahmed, O.A.; Balata, G.F.; Almalki, W.H. Self-assembled biodegradable polymeric micelles to improve dapoxetine delivery across the blood-brain barrier. Int. J. Nanomed. 2018, 13, 3679–3687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoo, H.S.; Park, T.G. Biodegradable polymeric micelles composed of doxorubicin conjugated PLGA–PEG block copolymer. J. Control Release 2001, 70, 63–70. [Google Scholar] [CrossRef]
- Riess, G. Micellization of block copolymers. Prog. Polym. Sci. 2003, 28, 1107–1170. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, O.A.; El-Say, K.M.; Aljaeid, B.M.; Badr-Eldin, S.M.; Ahmed, T.A. Optimized vinpocetine-loaded vitamin E D-α-tocopherol polyethylene glycol 1000 succinate-alpha lipoic acid micelles as a potential transdermal drug delivery system: In vitro and ex vivo studies. Int. J. Nanomed. 2019, 14, 33–43. [Google Scholar] [CrossRef] [Green Version]
- Gonda, A.; Zhao, N.; Shah, J.V.; Calvelli, H.R.; Kantamneni, H.; Francis, N.L.; Ganapathy, V. Engineering Tumor-Targeting Nanoparticles as Vehicles for Precision Nanomedicine. Med. One 2019, 4, e190021. [Google Scholar]
- Rejman, J.; Oberle, V.; Zuhorn, I.S.; Hoekstra, D. Size-dependent internalization of particles via the pathways of clathrin- and caveolae-mediated endocytosis. Biochem. J. 2004, 377, 159–169. [Google Scholar] [CrossRef]
- Mudalige, T.; Qu, H.; Van Haute, D.; Ansar, S.M.; Paredes, A.; Ingle, T. Chapter 11—Characterization of Nanomaterials: Tools and Challenges. In Micro and Nano Technologies; Rubio, A.L., Fabra Rovira, M.J., Martínez Sanz, M., Gómez-Mascaraque, L.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 313–353. ISBN 978-0-12-814130-4. [Google Scholar]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madhwi; Kumar, R.; Kumar, P.; Singh, B.; Sharma, G.; Katare, O.P.; Raza, K. In vivo pharmacokinetic studies and intracellular delivery of methotrexate by means of glycine-tethered PLGA-based polymeric micelles. Int. J. Pharm. 2017, 519, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Ashjari, M.; Khoee, S.; Mahdavian, A.R.; Rahmatolahzadeh, R. Self-assembled nanomicelles using PLGA–PEG amphiphilic block copolymer for insulin delivery: A physicochemical investigation and determination of CMC values. J. Mater. Sci. Mater. Med. 2012, 23, 943–953. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Yue, Y.; Maidana, D.E.; Bouzika, P.; Atik, A.; Matsumoto, H.; Miller, J.W.; Vavvas, D.G. Drug Delivery Nanoparticles: Toxicity Comparison in Retinal Pigment Epithelium and Retinal Vascular Endothelial Cells. Semin. Ophthalmol. 2016, 31, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Sai Lung, P.; Zhao, S.; Chu, Z.; Chrzanowski, W.; Li, Q. Shape dependent cytotoxicity of PLGA-PEG nanoparticles on human cells. Sci. Rep. 2017, 7, 7315. [Google Scholar] [CrossRef]
- Feuerecker, B.; Pirsig, S.; Seidl, C.; Aichler, M.; Feuchtinger, A.; Bruchelt, G.; Senekowitsch-Schmidtke, R. Lipoic acid inhibits cell proliferation of tumor cells in vitro and in vivo. Cancer Biol. Ther. 2012, 13, 1425–1435. [Google Scholar] [CrossRef] [Green Version]
- Na, M.H.; Seo, E.Y.; Kim, W.K. Effects of alpha-lipoic acid on cell proliferation and apoptosis in MDA-MB-231 human breast cells. Nutr. Res. Pract. 2009, 3, 265–271. [Google Scholar] [CrossRef]
- Carrillo-Castillo, T.D.; Castro-Carmona, J.S.; Luna-Velasco, A.; Zaragoza-Contreras, E.A. pH-responsive polymer micelles for methotrexate delivery at tumor microenvironments. E Polymers 2020, 20, 624–635. [Google Scholar] [CrossRef]
- Cheng, F.R.; Yang, Y.J.; Liang, Y.; Yan, J.Q.; Cao, J.; Su, T.; Jiang, L.; He, B.; Luo, X.L.; Gu, Z.W. Correlation of polymeric micelle sizes and their cellular internalization in vitro and tumor targeting in vivo. RSC Adv. 2014, 4, 62708–62716. [Google Scholar] [CrossRef]
- Wang, T.; Petrenko, V.A.; Torchilin, V.P. Paclitaxel-loaded polymeric micelles modified with MCF-7 cell-specific phage protein: Enhanced binding to target cancer cells and increased cytotoxicity. Mol. Pharm. 2010, 7, 1007–1014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakamuru, S.; Attene-Ramos, M.S.; Xia, M. Mitochondrial Membrane Potential Assay. Methods Mol. Biol. 2016, 1473, 17–22. [Google Scholar]
- Md, S.; Alhakamy, N.A.; Aldawsari, H.M.; Husain, M.; Kotta, S.; Abdullah, S.T.; Fahmy, U.A.; Alfaleh, M.A.; Asfour, H.Z. Formulation Design, Statistical Optimization, and In Vitro Evaluation of a Naringenin Nanoemulsion to Enhance Apoptotic Activity in A549 Lung Cancer Cells. Pharmaceuticals 2020, 13, 152. [Google Scholar] [CrossRef] [PubMed]
- Brentnall, M.; Rodriguez-Menocal, L.; de Guevara, R.L.; Cepero, E.; Boise, L.H. Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol. 2013, 14, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olszewski, M.B.; Groot, A.J.; Dastych, J.; Knol, E.F. TNF Trafficking to Human Mast Cell Granules: Mature Chain-Dependent Endocytosis. J. Immunol. 2007, 178, 5701–5709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez, L.; Yi, Y.; Yu, Y. Effect of partial PEGylation on particle uptake by macrophages. Nanoscale 2017, 9, 288–297. [Google Scholar] [CrossRef]
- Bent, R.; Moll, L.; Grabbe, S.; Bros, M. Interleukin-1 Beta-A Friend or Foe in Malignancies? Int. J. Mol. Sci. 2018, 19, 2155. [Google Scholar] [CrossRef] [Green Version]
- Cogswell, J.P.; Godlevski, M.M.; Wisely, G.B.; Clay, W.C.; Leesnitzer, L.M.; Ways, J.P.; Gray, J.G. NF-kappa B regulates IL-1 beta transcription through a consensus NF-kappa B binding site and a nonconsensus CRE-like site. J. Immunol. 1994, 153, 712–723. [Google Scholar] [PubMed]
- Cairns, J.A.; Guy, G.R.; Tan, Y.H. Interleukin-6 regulates the cytotoxic effect of tumour necrosis factor on U937 cells. Immunology 1992, 75, 669–673. [Google Scholar]
- Liu, B.; Qu, L.; Yan, S. Cyclooxygenase-2 promotes tumor growth and suppresses tumor immunity. Cancer Cell Int. 2015, 15, 106. [Google Scholar] [CrossRef] [Green Version]
- Khanzadeh, T.; Hagh, M.F.; Talebi, M.; Yousefi, B.; Azimi, A.; Hossein Pour Feizi, A.A.; Baradaran, B. Investigation of BAX and BCL2 expression and apoptosis in a resveratrol- and prednisolone-treated human T-ALL cell line, CCRF-CEM. Blood Res. 2018, 53, 53–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bresseleers, J.; Bagheri, M.; Storm, G.; Metselaar, J.M.; Hennink, W.E.; Meeuwissen, S.A.; van Hest, J.C.M. Scale-Up of the Manufacturing Process To Produce Docetaxel-Loaded mPEG-b-p(HPMA-Bz) Block Copolymer Micelles for Pharmaceutical Applications. Org. Process. Res. Dev. 2019, 23, 2707–2715. [Google Scholar] [CrossRef] [PubMed] [Green Version]











| Run | Factor Codes | Factor Values | ||||
|---|---|---|---|---|---|---|
| Factor-PEG-PLGA (PL) | Factor-Tween 80 (TW80) | Factor-Alpha-lipoic Acid (ALA) | Factor-PL (mg) | Factor-TW80 (%) | Factor-ALA (mg) | |
| 1 | 0 | 0 | 0 | 150 | 1.25 | 30 |
| 2 | 1 | 0 | 1 | 200 | 1.25 | 40 |
| 3 | 1 | −1 | 0 | 200 | 0.5 | 30 |
| 4 | −1 | 1 | 0 | 100 | 2 | 30 |
| 5 | 0 | −1 | 1 | 150 | 0.5 | 40 |
| 6 | −1 | 0 | −1 | 100 | 1.25 | 20 |
| 7 | −1 | −1 | 0 | 100 | 0.5 | 30 |
| 8 | 1 | 0 | −1 | 200 | 1.25 | 20 |
| 9 | 0 | 0 | 0 | 150 | 1.25 | 30 |
| 10 | 1 | 1 | 0 | 200 | 2 | 30 |
| 11 | 0 | 0 | 0 | 150 | 1.25 | 30 |
| 12 | −1 | 0 | 1 | 100 | 1.25 | 40 |
| 13 | 0 | 1 | −1 | 150 | 2 | 20 |
| 14 | 0 | −1 | −1 | 150 | 0.5 | 20 |
| 15 | 0 | 1 | 1 | 150 | 2 | 40 |
| Bax F | 5’-TGGCAGCTGACATGTTTTCTGAC-3’ |
| Bax R | 5’-TCACCCAACCACCCTGGTCTT-3’ |
| Bcl-2 F | 5’-TCGCCCTGTGGATGACTGA-3’ |
| Bcl-2 R | 5’-CAGAGACAGCCAGGAGAAATCA-3’ |
| GAPDH F | 5’-AATGCATCCTGCACCACCAA-3’ |
| GAPDH R | 5’-GATGCCATATTCATTGTCATA-3’ |
| Run | Independent Factors | Dependent Factor | |||
|---|---|---|---|---|---|
| Factor-PEG-PLGA (PL) (mg) | Factor-Tween 80 (TW80) (%) | Factor-Alpha-lipoic Acid (ALA) (mg) | Mean Particle Size (PS) (nm) | ||
| Observed Values | Fitted Values | ||||
| 1 | 150 | 1.25 | 30 | 216 | 216.0 |
| 2 | 200 | 1.25 | 40 | 226 | 202.0 |
| 3 | 200 | 0.5 | 30 | 194 | 218.0 |
| 4 | 100 | 2 | 30 | 214 | 216.0 |
| 5 | 150 | 0.5 | 40 | 181 | 191.25 |
| 6 | 100 | 1.25 | 20 | 98 | 84.25 |
| 7 | 100 | 0.5 | 30 | 342 | 355.75 |
| 8 | 200 | 1.25 | 20 | 141 | 168.25 |
| 9 | 150 | 1.25 | 30 | 395 | 378.0 |
| 10 | 200 | 2 | 30 | 218 | 216.0 |
| 11 | 150 | 1.25 | 30 | 106 | 102.75 |
| 12 | 100 | 1.25 | 40 | 126 | 115.75 |
| 13 | 150 | 2 | 20 | 387 | 390.25 |
| 14 | 150 | 0.5 | 20 | 287 | 259.75 |
| 15 | 150 | 2 | 40 | 165 | 182.0 |
| Source | Sum of Squares | Degrees of Freedom | Mean Square | F-Ratio | p-Value |
|---|---|---|---|---|---|
| A: PEG-PLGA (PL) | 65,884.5 | 1 | 65,884.5 | 85.95 | 0.0002 |
| B: Tween 80 (TW80) | 16,200.0 | 1 | 16,200.0 | 21.14 | 0.0059 |
| C: Alpha-lipoic acid (ALA) | 22,472.0 | 1 | 22,472.0 | 29.32 | 0.0029 |
| AA | 1787.08 | 1 | 1787.08 | 2.33 | 0.1873 |
| AB | 36.0 | 1 | 36.0 | 0.05 | 0.8370 |
| AC | 8649.0 | 1 | 8649.0 | 11.28 | 0.0201 |
| BB | 1953.23 | 1 | 1953.23 | 2.55 | 0.1713 |
| BC | 4900.0 | 1 | 4900.0 | 6.39 | 0.0526 |
| CC | 132.923 | 1 | 132.923 | 0.17 | 0.6944 |
| Total error | 3832.5 | 5 | 766.5 | --- | --- |
| Total (corr.) | 126,157 | 14 | --- | --- | --- |
| Factor | Low | High | Optimum |
|---|---|---|---|
| PEG-PLGA (PL) (mg) | 100 | 200 | 100.282 |
| Tween 80 (TW80) (%) | 0.5 | 2.0 | 2.0 |
| Alpha-lipoic acid (ALA) (mg) | 20 | 40 | 40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhakamy, N.A.; Ahmed, O.A.A.; Fahmy, U.A.; Md, S. Development and In Vitro Evaluation of 2-Methoxyestradiol Loaded Polymeric Micelles for Enhancing Anticancer Activities in Prostate Cancer. Polymers 2021, 13, 884. https://doi.org/10.3390/polym13060884
Alhakamy NA, Ahmed OAA, Fahmy UA, Md S. Development and In Vitro Evaluation of 2-Methoxyestradiol Loaded Polymeric Micelles for Enhancing Anticancer Activities in Prostate Cancer. Polymers. 2021; 13(6):884. https://doi.org/10.3390/polym13060884
Chicago/Turabian StyleAlhakamy, Nabil A., Osama A. A. Ahmed, Usama A. Fahmy, and Shadab Md. 2021. "Development and In Vitro Evaluation of 2-Methoxyestradiol Loaded Polymeric Micelles for Enhancing Anticancer Activities in Prostate Cancer" Polymers 13, no. 6: 884. https://doi.org/10.3390/polym13060884