Synthesis of Retinol-Loaded Lipid Nanocarrier via Vacuum Emulsification to Improve Topical Skin Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
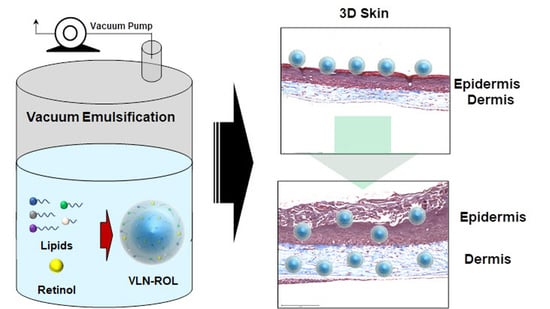
2.2. Synthesis of Retinol-Loaded Lipid Nanoparticles
2.3. Properties of Retinol-Loaded Lipid Nanoparticles
2.3.1. Stability Study of Retinol in Retinol-Loaded Lipid Nanoparticles
2.3.2. Particle Size and Zeta Potential Analysis of Retinol-Loaded Lipid Nanoparticles
2.3.3. Differential Scanning Calorimetric (DSC) Analysis of Retinol-Loaded Lipid Nanoparticles
2.4. Skin Penetration Analysis
2.5. Reconstructed 3D Human Skin
2.6. Statistical Analysis
3. Results and Discussion
3.1. Synthesis of Retinol-Loaded Lipid Nanoparticles via Vacuum Emulsification
3.2. Optimization of Composition of Retinol-Loaded Lipid Nanoparticles
3.2.1. Effect of Lipid Composition
3.2.2. Effect of Surfactant
3.2.3. Effect of pH
3.3. DSC Analysis of Retinol-Loaded Lipid Nanoparticles
3.4. Penetration Study of Retinol-Loaded Lipid Nanoparticles on Porcine Skin
3.5. Effects of Retinol-Loaded Lipid Nanoparticles on 3D Skin Model
3.6. Practical Application and Future Research Perspectives
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mukherjee, S.; Date, A.; Patravale, V.; Korting, H.C.; Roeder, A.; Weindl, G. Retinoids in the treatment of skin aging: An overview of clinical efficacy and safety. Clin. Interv. Aging 2006, 1, 327–348. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, C.E.M. The role of retinoids in the prevention and repair of aged and photoaged skin. Clin. Exp. Dermatol. 2001, 26, 613–618. [Google Scholar] [CrossRef]
- Stratigos, A.J.; Katsambas, A.D. The Role of Topical Retinoids in the Treatment of Photoaging. Drugs 2005, 65, 1061–1072. [Google Scholar] [CrossRef] [PubMed]
- Riahi, R.R.; Bush, A.E.; Cohen, P.R. Topical Retinoids: Therapeutic Mechanisms in the Treatment of Photodamaged Skin. Am. J. Clin. Dermatol. 2016, 17, 265–276. [Google Scholar] [CrossRef]
- Saurat, J.-H. Side effects of systemic retinoids and their clinical management. J. Am. Acad. Dermatol. 1992, 27, S23–S28. [Google Scholar] [CrossRef]
- Lee, S.-C.; Yuk, H.-G.; Lee, N.-H.; Lee, K.-E.; Hwang, Y.-I.; Ludescher, R.D. Stabilization of retinol through incorporation into liposomes. J. Biochem. Mol. Biol. 2002, 35, 358–363. [Google Scholar] [PubMed]
- Truchuelo, M.T.; Jimenez, N.; Miguel-Gomez, L.; Hermosa, A.; Sánchez-Neila, N.; Cuevas, J. Histological and Immunohistochemical Evaluation of the Efficacy of a New Cosmetic Formulation in the Treatment of Skin Photoaging. Dermatol. Res. Pract. 2017, 2017, 8407247. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Kim, N.; Jung, S.; Mun, J.; Kim, J.; Kim, B.; Lee, J.; Ryoo, H.; Jung, H. Improvement in skin wrinkles from the use of photostable retinyl retinoate: A randomized controlled trial. Br. J. Dermatol. 2009, 162, 497–502. [Google Scholar] [CrossRef]
- Kim, H.; Kim, B.; Kim, H.; Um, S.; Lee, J.; Ryoo, H.; Jung, H. Synthesis and in vitro biological activity of retinyl retinoate, a novel hybrid retinoid derivative. Bioorg. Med. Chem. 2008, 16, 6387–6393. [Google Scholar] [CrossRef]
- O’Byrne, S.M.; Blaner, W.S. Retinol and retinyl esters: Biochemistry and physiology: Thematic Review Series: Fat-Soluble Vitamins: Vitamin A. J. Lipid Res. 2013, 54, 1731–1743. [Google Scholar] [CrossRef] [Green Version]
- Chmykh, Y.G.; Nadeau, J.L. Characterization of Retinol Stabilized in Phosphatidylcholine Vesicles with and without Antioxidants. ACS Omega 2020, 5, 18367–18375. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Mun, S.; Kim, Y.-R. UV and storage stability of retinol contained in oil-in-water nanoemulsions. Food Chem. 2019, 272, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Goudon, F.; Clément, Y.; Ripoll, L. Controlled Release of Retinol in Cationic Co-Polymeric Nanoparticles for Topical Application. Cosmetics 2020, 7, 29. [Google Scholar] [CrossRef]
- Park, C.-E.; Park, D.-J.; Kim, B.-K. Effects of a chitosan coating on properties of retinol-encapsulated zein nanoparticles. Food Sci. Biotechnol. 2015, 24, 1725–1733. [Google Scholar] [CrossRef]
- Pardeike, J.; Hommoss, A.; Müller, R.H. Lipid nanoparticles (SLN, NLC) in cosmetic and pharmaceutical dermal products. Int. J. Pharm. 2009, 366, 170–184. [Google Scholar] [CrossRef]
- Puglia, C.; Bonina, F. Lipid nanoparticles as novel delivery systems for cosmetics and dermal pharmaceuticals. Expert Opin. Drug Deliv. 2012, 9, 429–441. [Google Scholar] [CrossRef]
- Ding, Y.; Pyo, S.M.; Müller, R.H. smartLipids® as third solid lipid nanoparticle generation—Stabilization of retinol for dermal application. Die Pharm. 2017, 72, 728–735. [Google Scholar]
- Severino, P.; Santana, M.H.A.; Souto, E.B. Optimizing SLN and NLC by 22 full factorial design: Effect of homogenization technique. Mater. Sci. Eng. C 2012, 32, 1375–1379. [Google Scholar] [CrossRef]
- Souto, E.B.; Müller, R.H. Investigation of the factors influencing the incorporation of clotrimazole in SLN and NLC prepared by hot high-pressure homogenization. J. Microencapsul. 2006, 23, 377–388. [Google Scholar] [CrossRef]
- Zhuang, C.-Y.; Li, N.; Wang, M.; Zhang, X.-N.; Pan, W.-S.; Peng, J.-J.; Pan, Y.-S.; Tang, X. Preparation and characterization of vinpocetine loaded nanostructured lipid carriers (NLC) for improved oral bioavailability. Int. J. Pharm. 2010, 394, 179–185. [Google Scholar] [CrossRef]
- Mehnert, W.; Mäder, K. Solid lipid nanoparticles. Adv. Drug Deliv. Rev. 2012, 64, 83–101. [Google Scholar] [CrossRef]
- Hu, F.-Q.; Jiang, S.-P.; Du, Y.-Z.; Yuan, H.; Ye, Y.-Q.; Zeng, S. Preparation and characterization of stearic acid nanostructured lipid carriers by solvent diffusion method in an aqueous system. Colloids Surf. B Biointerfaces 2005, 45, 167–173. [Google Scholar] [CrossRef]
- Shete, H.; Patravale, V. Long chain lipid based tamoxifen NLC. Part I: Preformulation studies, formulation development and physicochemical characterization. Int. J. Pharm. 2013, 454, 573–583. [Google Scholar] [CrossRef]
- Souto, E.B.; Müller, R.H. SLN and NLC for topical delivery of ketoconazole. J. Microencapsul. 2005, 22, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Mottaleb, M.M.; Neumann, D.; Lamprecht, A. Lipid nanocapsules for dermal application: A comparative study of lipid-based versus polymer-based nanocarriers. Eur. J. Pharm. Biopharm. 2011, 79, 36–42. [Google Scholar] [CrossRef]
- Sun, M.; Nie, S.; Pan, X.; Zhang, R.; Fan, Z.; Wang, S. Quercetin-nanostructured lipid carriers: Characteristics and anti-breast cancer activities in vitro. Colloids Surf. B Biointerfaces 2014, 113, 15–24. [Google Scholar] [CrossRef]
- Couvreur, P.; Blanco-Prieto, M.J.; Puisieux, F.; Roques, B.; Fattal, E. Multiple emulsion technology for the design of microspheres containing peptides and oligopeptides. Adv. Drug Deliv. Rev. 1997, 28, 85–96. [Google Scholar] [CrossRef]
- Demurtas, A.; Pescina, S.; Nicoli, S.; Santi, P.; Padula, C. Development and validation of a simple method for the extraction and quantification of crisaborole in skin layers. Biomed. Chromatogr. 2019, 33, e4664. [Google Scholar] [CrossRef] [PubMed]
- Tantikarnjathep, K.; Sebranek, J.G.; Topel, D.G.; Rust, R.E. Use of Vacuum During Formation of Meat Emulsions. J. Food Sci. 1983, 48, 1039–1041. [Google Scholar] [CrossRef]
- Weber, S.; Zimmer, A.; Pardeike, J. Solid Lipid Nanoparticles (SLN) and Nanostructured Lipid Carriers (NLC) for pulmonary application: A review of the state of the art. Eur. J. Pharm. Biopharm. 2014, 86, 7–22. [Google Scholar] [CrossRef]
- Han, F.; Li, S.; Yin, R.; Liu, H.; Xu, L. Effect of surfactants on the formation and characterization of a new type of colloidal drug delivery system: Nanostructured lipid carriers. Colloids Surf. A Physicochem. Eng. Asp. 2008, 315, 210–216. [Google Scholar] [CrossRef]
- Aljaeid, B.M.; Hosny, K.M. Miconazole-loaded solid lipid nanoparticles: Formulation and evaluation of a novel formula with high bioavailability and antifungal activity. Int. J. Nanomed. 2016, 11, 441–447. [Google Scholar] [CrossRef] [Green Version]
- Almeida, H.; Amaral, M.H.; Lobão, P. Comparative study of sustained-release lipid microparticles and solid dispersions containing ibuprofen. Braz. J. Pharm. Sci. 2012, 48, 529–536. [Google Scholar] [CrossRef] [Green Version]
- Gönüllü, Ü.; Üner, M.; Yener, G.; Karaman, E.F.; Aydoğmuş, Z. Formulation and characterization of solid lipid nanoparticles, nanostructured lipid carriers and nanoemulsion of lornoxicam for transdermal delivery. Acta Pharm. 2015, 65, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Teeranachaideekul, V.; Müller, R.H.; Junyaprasert, V.B. Encapsulation of ascorbyl palmitate in nanostructured lipid carriers (NLC)—Effects of formulation parameters on physicochemical stability. Int. J. Pharm. 2007, 340, 198–206. [Google Scholar] [CrossRef]
- Eskandar, N.G.; Simovic, S.; Prestidge, C.A. Nanoparticle Coated Submicron Emulsions: Sustained In-vitro Release and Improved Dermal Delivery of All-trans-retinol. Pharm. Res. 2009, 26, 1764–1775. [Google Scholar] [CrossRef] [PubMed]
- Jenning, V.; Gysler, A.; Schäfer-Korting, M.; Gohla, S.H. Vitamin A loaded solid lipid nanoparticles for topical use: Occlusive properties and drug targeting to the upper skin. Eur. J. Pharm. Biopharm. 2000, 49, 211–218. [Google Scholar] [CrossRef]
- Müller, R.H.; Olechowski, F.; Köpke, D.; Pyo, S.M. SmartLipids—The Third Generation of Solid Submicron Lipid Particles for Dermal Delivery of Actives. In Nanocosmetics: From Ideas to Products; Cornier, J., Keck, C.M., van de Voorde, M., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 141–159. [Google Scholar]
- Jensen, L.E. Targeting the IL-1 family members in skin inflammation. Curr. Opin. Investig. Drugs 2010, 11, 1211–1220. [Google Scholar] [PubMed]
- SanMiguel, J.C.; Olaru, F.; Li, J.; Mohr, E.; Jensen, L.E. Interleukin-1 regulates keratinocyte expression of T cell targeting chemokines through interleukin-1 receptor associated kinase-1 (IRAK1) dependent and independent pathways. Cell. Signal. 2009, 21, 685–694. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Formula | #1 | #2 | #3 | #4 | #5 | |
|---|---|---|---|---|---|---|
| Weight content | ||||||
| Solid lipids | Glyceryl behenate | 3.6% | 3.6% | 3.6% | 3.6% | 3.0% |
| Stearylamine | 2.4% | |||||
| Glyceryl stearate | 0.6% | 0.6% | 0.6% | 0.8% | ||
| Glyceryl distearate | 0.6% | 0.6% | 0.6% | 0.8% | ||
| Cetyl palmitate | 0.6% | 0.6% | 0.6% | 0.8% | ||
| Myristyl Myristate | 0.6% | 0.6% | 0.6% | 0.6% | ||
| Liquid lipids | Caprylic/Capric Triglyceride | 3.0% | 2.4% | 2.4% | 2.4% | 2.4% |
| Retinol | 3.0% | 3.0% | 3.0% | 3.0% | 3.0% | |
| Surfactants | Polysorbate 60 | 1.8% | 0.7% | 0.7% | 0.7% | 0.7% |
| Poloxamer 188 | 0.7% | 0.7% | 0.7% | 0.7% | ||
| Hydrogenated lecithin | 0.2% | 0.2% | 0.2% | 0.2% | ||
| Anionic surfactant | 0.2% | 0.2% | 0.6% | 1.2% | 0.6% | |
| DI | up to 100 | |||||
| Surfactant Con.(%) | Vacuum Emulsification | Normal Emulsification | ||
|---|---|---|---|---|
| Z-ave (nm) | PDI | Z-ave (nm) | PDI | |
| 10 | 218.6 | 0.183 | 2416 | 0.593 |
| 5 | 242.0 | 0.209 | Phase separation | |
| 2 | 405.1 | 0.172 | Phase separation | |
| Formula | Surfactant | Size (nm) | PDI | Zeta Potential (mV) |
|---|---|---|---|---|
| #1 | - | 1386 | 0.95 | 12.9 |
| #2 | CP | 303.5 | 0.216 | −48.7 |
| #3 | 319.3 | 0.217 | −51.4 | |
| #4 | 306.1 | 0.196 | −54.9 | |
| #2 | SSG | 294.0 | 0.221 | −47.9 |
| #3 | 269.7 | 0.201 | −54.6 | |
| #4 | 257.9 | 0.205 | −62.2 | |
| #5 | 278.1 | 0.191 | −50.9 |
| Size (nm) | Distribution (PDI) | Zeta Potential (mV) | Thermal Stability (4 Weeks) | ||
|---|---|---|---|---|---|
| 25 °C | 40 °C | 50 °C | |||
| 158.4 | 0.27 | −53.1 ± 3.24 | 97% | 92% | 92% |
| Sample | T1(°C) | T2(°C) | H1 (J/g) | H2 (J/g) |
|---|---|---|---|---|
| 1 | 53.6 | 67.7 | 0.23 | 10.25 |
| 3 | 53.9 | 61.9 | 0.82 | 4.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jun, S.-H.; Kim, H.; Lee, H.; Song, J.E.; Park, S.G.; Kang, N.-G. Synthesis of Retinol-Loaded Lipid Nanocarrier via Vacuum Emulsification to Improve Topical Skin Delivery. Polymers 2021, 13, 826. https://doi.org/10.3390/polym13050826
Jun S-H, Kim H, Lee H, Song JE, Park SG, Kang N-G. Synthesis of Retinol-Loaded Lipid Nanocarrier via Vacuum Emulsification to Improve Topical Skin Delivery. Polymers. 2021; 13(5):826. https://doi.org/10.3390/polym13050826
Chicago/Turabian StyleJun, Seung-Hyun, Hanul Kim, HyeJin Lee, Ji Eun Song, Sun Gyoo Park, and Nea-Gyu Kang. 2021. "Synthesis of Retinol-Loaded Lipid Nanocarrier via Vacuum Emulsification to Improve Topical Skin Delivery" Polymers 13, no. 5: 826. https://doi.org/10.3390/polym13050826