Chitosan Nanocarrier Entrapping Hydrophilic Drugs as Advanced Polymeric System for Dual Pharmaceutical and Cosmeceutical Application: A Comprehensive Analysis Using Box-Behnken Design
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
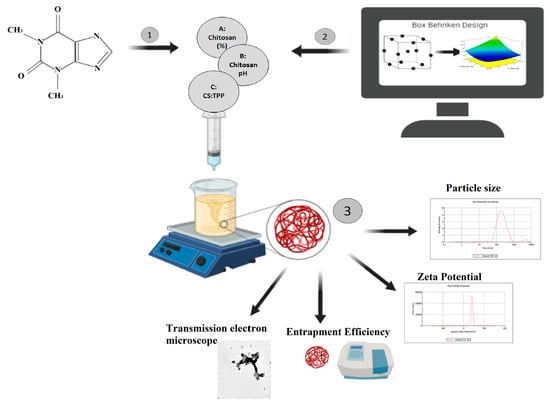
2.2.1. Experiment Design
2.2.2. Fitting of Responses to Optimum Model
2.2.3. Preparation of Caffeine-Loaded CS–TPP Nanoparticles by Ionic Gelation Technique
2.2.4. Characterization of Caffeine-Loaded CS–TPP Nanoparticles
Entrapment Efficiency Percent (EE%)
Particle Size (PS) and Polydispersity Index (PDI)
Zeta Potential (ZP)
2.2.5. Formulation Optimization
2.2.6. Characterization of the Optimal-Caffeine Loaded CS–TPP Nanoparticles Formulation
Entrapment Efficiency, Particle Size, Polydispersity Index, and Zeta Potential
Transmission Electron Microscope (TEM) Examination
3. Results and Discussion
3.1. Effect of Investigated Independent Variables on Entrapment Efficiency Percent (EE%)
3.2. Effect of the Investigated Independent Variables on Particle Size (PS)
3.3. Effect of the Investigated Independent Variables on Polydispersity Index (PDI)
3.4. Effect of the Investigated Independent Variables on Zeta Potential (ZP)
3.5. Formulation Optimization
3.6. Characterization of the Optimal Caffeine-Loaded CS–TPP Nanoparticles
3.6.1. Determination entrapment Efficiency (EE%), Particle Size (PS), Polydispersity Index (PDI), and Zeta Potential (ZP)
3.6.2. Transmission Electron Microscope (TEM) Examination
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Rawlings, A.V. Cellulite and its treatment. Int. J. Cosmet. Sci. 2006, 28, 175–190. [Google Scholar] [CrossRef]
- Khan, M.H.; Victor, F.; Rao, B.; Sadick, N.S. Treatment of cellulite: Part I. Pathophysiology. J. Am. Acad. Dermatol. 2010, 62, 361–370. [Google Scholar] [CrossRef]
- Harpaz, E.; Tamir, S.; Weinstein, A.; Weinstein, Y. The effect of caffeine on energy balance. J. Basic Clin. Physiol. Pharmacol. 2017, 28, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Abosabaa, S.A.; Arafa, M.G.; ElMeshad, A.N. Drug delivery systems integrated with conventional and advanced treatment approaches toward cellulite reduction. J. Drug Deliv. Sci. Technol. 2020, 60, 102084. [Google Scholar] [CrossRef]
- Vogelgesang, B.; Bonnet, I.; Godard, N.; Sohm, B.; Perrier, E. In vitro and in vivo efficacy of sulfo-carrabiose, a sugar-based cosmetic ingredient with anti-cellulite properties. Int. J. Cosmet. Sci. 2010, 33, 120–125. [Google Scholar] [CrossRef]
- Diepvens, K.; Westerterp, K.R.; Westerterp-Plantenga, M.S. Obesity and thermogenesis related to the consumption of caffeine, ephedrine, capsaicin, and green tea. Am. J. Physiol. Integr. Comp. Physiol. 2007, 292, R77–R85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dodd, S.L.; Herb, R.A.; Powers, S.K.; Dodd, S.L.; Dodd, S.L. Caffeine and Exercise Performance. Sports Med. 1993, 15, 14–23. [Google Scholar] [CrossRef]
- Panchal, S.K.; Poudyal, H.; Waanders, J.; Brown, L. Coffee Extract Attenuates Changes in Cardiovascular and Hepatic Structure and Function without Decreasing Obesity in High-Carbohydrate, High-Fat Diet-Fed Male Rats. J. Nutr. 2012, 142, 690–697. [Google Scholar] [CrossRef] [Green Version]
- Javadzadeh, Y.; Bahari, L.A. Therapeutic nanostructures for dermal and transdermal drug delivery. In Nano-and Microscale Drug Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2017; pp. 131–146. [Google Scholar]
- Wu, X.; Landfester, K.; Musyanovych, A.; Guy, R. Disposition of Charged Nanoparticles after Their Topical Application to the Skin. Ski. Pharmacol. Physiol. 2010, 23, 117–123. [Google Scholar] [CrossRef]
- Arafa, M.G.; Ayoub, B.M. Bioavailability Study of Niosomal Salbutamol Sulfate in Metered Dose Inhaler: Controlled Pulmonary Drug Delivery. J. Aerosol Med. Pulm. Drug Deliv. 2018, 31, 114–115. [Google Scholar] [CrossRef]
- Vyas, L.K.; Tapar, K.K.; Nema, R.K.; Parashar, A.K. Development and characterization of topical liposomal gel formulation for anti-cellulite activity. Measurement 2013, 15, 18. [Google Scholar]
- Iskandarsyah, I.; Puteri, A.W.; Ernysagita, E. Penetration test of caffeine in ethosome and desmosome gel using an in vitro method. Int. J. Appl. Pharm. 2017, 9, 120–123. [Google Scholar] [CrossRef] [Green Version]
- Algul, D.; Duman, G.; Ozdemir, S.; Acar, E.T.; Yener, G. Preformulation, Characterization, and In Vitro Release Studies of Caffeine-Loaded Solid Lipid Nanoparticles. J. Cosmet. Sci. 2018, 69, 165–173. [Google Scholar]
- Massella, D.; Celasco, E.; Salaün, F.; Ferri, A.; Barresi, A.A. Overcoming the limits of flash nanoprecipitation: Effective loading of hydrophilic drug into polymeric nanoparticles with controlled structure. Polymers 2018, 10, 1092. [Google Scholar] [CrossRef] [Green Version]
- Abu Hassan, N.A.F.; Sahudin, S.; Hussain, Z.; Hussain, M. Self-assembled chitosan nanoparticles for percutaneous delivery of caffeine: Preparation, characterization and in vitro release studies. Int. J. Appl. Pharm. 2018, 10, 172–185. [Google Scholar] [CrossRef]
- Suptijah, P.; Djajadisastra, J.; Saputro, C.K.H.; Hidayat, T. The Characterization and Effectiveness Penetrasion of Caffeine Trapped and Coated Chitosan Nanoparticles as Anti-Cellulite. J. Nanosci. Nanoeng. 2015, 1, 198–205. [Google Scholar]
- Zhang, Z.; Tsai, P.-C.; Ramezanli, T.; Michniak-Kohn, B.B. Polymeric nanoparticles-based topical delivery systems for the treatment of dermatological diseases. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2013, 5, 205–218. [Google Scholar] [CrossRef] [Green Version]
- Fereig, S.A.; El-Zaafarany, G.M.; Arafa, M.G.; Abdel-Mottaleb, M.M.A. Tackling the various classes of nano-therapeutics employed in topical therapy of psoriasis. Drug Deliv. 2020, 27, 662–680. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Hwang, Y.; Oh, H.; Kim, S.; Kim, J.-H.; Lee, J.-H.; Shin, Y.C.; Tae, G.; Choi, W.I. A novel chitosan nanocapsule for enhanced skin penetration of cyclosporin A and effective hair growth in vivo. Nano Res. 2019, 12, 3024–3030. [Google Scholar] [CrossRef]
- Tu, Y.; Wang, X.; Lu, Y.; Zhang, H.; Yu, Y.; Chen, Y.; Liu, J.; Sun, Z.; Cui, L.; Gao, J.; et al. Promotion of the transdermal delivery of protein drugs by N-trimethyl chitosan nanoparticles combined with polypropylene electret. Int. J. Nanomed. 2016, 11, 5549–5561. [Google Scholar] [CrossRef] [Green Version]
- Calvo, P.; Vila-Jato, J.L.; Alonso, M.J. Novel hydrophilic chitosan-polyethylene oxide nanoparticles as protein carriers. J. Appl. Polym. Sci. 1997, 63, 125–132. [Google Scholar] [CrossRef]
- Abd-Allah, H.; Abdel-Aziz, R.T.; Nasr, M. Chitosan nanoparticles making their way to clinical practice: A feasibility study on their topical use for acne treatment. Int. J. Biol. Macromol. 2020, 156, 262–270. [Google Scholar] [CrossRef]
- Delan, W.K.; Zakaria, M.; Elsaadany, B.; ElMeshad, A.N.; Mamdouh, W.; Fares, A.R. Formulation of simvastatin chitosan nanoparticles for controlled delivery in bone regeneration: Optimization using Box-Behnken design, stability and in vivo study. Int. J. Pharm. 2020, 577, 119038. [Google Scholar] [CrossRef]
- Thandapani, G.; Prasad, S.; Sudha, P. Sukumaran, Size optimization and in vitro biocompatibility studies of chitosan nanoparticles. Int. J. Biol. Macromol. 2017, 104, 1794–1806. [Google Scholar] [CrossRef]
- Dudhani, A.R.; Kosaraju, S.L. Bioadhesive chitosan nanoparticles: Preparation and characterization. Carbohydr. Polym. 2010, 81, 243–251. [Google Scholar] [CrossRef]
- Servat-Medina, L.; González-Gómez, A.; Reyes-Ortega, F.; Sousa, I.M.O.; Queiroz, N.D.C.A.; Zago, P.M.W.; Jorge, M.P.; Monteiro, K.M.; de Carvalho, J.E.; Román, J.S. Chitosan–tripolyphosphate nanoparticles as Arrabidaea chica standardized extract carrier: Synthesis, characterization, biocompatibility, and antiulcerogenic activity. Int. J. Nanomed. 2015, 10, 3897. [Google Scholar] [CrossRef] [Green Version]
- Sobhani, Z.; Samani, S.M.; Montaseri, H.; Khezri, E. Nanoparticles of Chitosan Loaded Ciprofloxacin: Fabrication and Antimicrobial Activity. Adv. Pharm. Bull. 2017, 7, 427–432. [Google Scholar] [CrossRef] [Green Version]
- Calderon, L.; Harris, R.; Cordoba-Diaz, M.; Elorza, M.A.; Elorza, B.; Lenoir, J.; Adriaens, E.; Remon, J.; Heras, A.; Cordoba-Diaz, D.; et al. Nano and microparticulate chitosan-based systems for antiviral topical delivery. Eur. J. Pharm. Sci. 2013, 48, 216–222. [Google Scholar] [CrossRef]
- Sabra, R.; Billa, N.; Roberts, C.J. An augmented delivery of the anticancer agent, curcumin, to the colon. React. Funct. Polym. 2018, 123, 54–60. [Google Scholar] [CrossRef]
- Mujtaba, A.; Ali, M.; Kohli, K. Statistical optimization and characterization of pH-independent extended-release drug delivery of cefpodoxime proxetil using Box–Behnken design. Chem. Eng. Res. Design 2014, 92, 156–165. [Google Scholar] [CrossRef]
- Issa, M.A.; Abidin, Z.Z.; Sobri, S.; Abdul-Rashid, S.; Mahdi, M.A.; Ibrahim, N.A.; Pudza, M.Y. Fabrication, characterization and response surface method optimization for quantum efficiency of fluorescent nitrogen-doped carbon dots obtained from carboxymethylcellulose of oil palms empty fruit bunch. Chin. J. Chem. Eng. 2020, 28, 584–592. [Google Scholar] [CrossRef]
- Chopra, S.; Motwani, S.K.; Iqbal, Z.; Talegaonkar, S.; Ahmad, F.J.; Khar, R.K.; Talegaonkar, S. Optimisation of polyherbal gels for vaginal drug delivery by Box-Behnken statistical design. Eur. J. Pharm. Biopharm. 2007, 67, 120–131. [Google Scholar] [CrossRef]
- Hashad, R.A.; Ishak, R.A.; Geneidi, A.S.; Mansour, S. Methotrexate loading in chitosan nanoparticles at a novel pH: Response surface modeling, optimization and characterization. Int. J. Biol. Macromol. 2016, 91, 630–639. [Google Scholar] [CrossRef]
- Hashad, R.A.; Ishak, R.A.; Fahmy, S.; Mansour, S.; Geneidi, A.S. Chitosan-tripolyphosphate nanoparticles: Optimization of formulation parameters for improving process yield at a novel pH using artificial neural networks. Int. J. Biol. Macromol. 2016, 86, 50–58. [Google Scholar] [CrossRef]
- Shah, B.; Khunt, D.; Misra, M.; Padh, H. Application of Box-Behnken design for optimization and development of quetiapine fumarate loaded chitosan nanoparticles for brain delivery via intranasal route. Int. J. Biol. Macromol. 2016, 89, 206–218. [Google Scholar] [CrossRef]
- Ghosal, K.; Ghosh, D.; Das, S.K. Preparation and evaluation of naringin-loaded polycaprolactone microspheres based oral suspension using Box-Behnken design. J. Mol. Liq. 2018, 256, 49–57. [Google Scholar] [CrossRef]
- Khan, N.; Alruwaili, N.K.; Bukhari, S.N.A.; Alsuwayt, B.; Afzal, M.; Akhter, S.; Yasir, M.; Elmowafy, M.; Shalaby, K.; Ali, A. Improvement of Ocular Efficacy of Levofloxacin by Bioadhesive Chitosan Coated PLGA Nanoparticles: Box-behnken Design, In-vitro Characterization, Antibacterial Evaluation and Scintigraphy Study. Antibact. Eval. Scintigr. Study Iran. J. Pharm. Res. 2020, 19, 292–311. [Google Scholar]
- Sathyamoorthy, N.; Magharla, D.; Chintamaneni, P.; Vankayalu, S. Optimization of paclitaxel loaded poly (ε-caprolactone) nanoparticles using Box Behnken design. Beni Suef Univ. J. Basic Appl. Sci. 2017, 6, 362–373. [Google Scholar] [CrossRef]
- Isam, M.; Baloo, L.; Kutty, S.R.M.; Yavari, S. Optimisation and Modelling of Pb (II) and Cu (II) Biosorption onto Red Algae (Gracilaria changii) by Using Response Surface Methodology. Water 2019, 11, 2325. [Google Scholar] [CrossRef] [Green Version]
- Motawi, T.K.; El-Maraghy, S.A.; ElMeshad, A.N.; Nady, O.M.; Hammam, O.A. Cromolyn chitosan nanoparticles as a novel protective approach for colorectal cancer. Chem. Interact. 2017, 275, 1–12. [Google Scholar] [CrossRef]
- Fazil, M.; Md, S.; Haque, S.; Kumar, M.; Baboota, S.; Sahni, J.K.; Ali, J. Development and evaluation of rivastigmine loaded chitosan nanoparticles for brain targeting. Eur. J. Pharm. Sci. 2012, 47, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Arafa, M.G.; Mousa, H.A.; Afifi, N.N. Preparation of PLGA-chitosan based nanocarriers for enhancing antibacterial effect of ciprofloxacin in root canal infection. Drug Deliv. 2019, 27, 26–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Gao, C. Preparation and properties of ionically cross-linked chitosan nanoparticles. Polym. Adv. Technol. 2008, 20, 613–619. [Google Scholar] [CrossRef]
- Honary, S.; Zahir, F. Effect of zeta potential on the properties of nano-drug delivery systems-a review (Part 2). Trop. J. Pharm. Res. 2013, 12, 265–273. [Google Scholar]
- Sun, D.; Kang, S.; Liu, C.; Lu, Q.; Cui, L.; Hu, B. Effect of zeta potential and particle size on the stability of SiO2 nanospheres as carrier for ultrasound imaging contrast agents. Int. J. Electrochem. Sci. 2016, 11, 8520–8529. [Google Scholar] [CrossRef]
- Kalam, M.A.; Khan, A.A.; Khan, S.; Almalik, A.; Alshamsan, A. Optimizing indomethacin-loaded chitosan nanoparticle size, encapsulation, and release using Box–Behnken experimental design. Int. J. Biol. Macromol. 2016, 87, 329–340. [Google Scholar] [CrossRef]
- Bodmeier, R.; Oh, K.-H.; Pramar, Y. Preparation and Evaluation of Drug-Containing Chitosan Beads. Drug Dev. Ind. Pharm. 1989, 15, 1475–1494. [Google Scholar] [CrossRef]
- Lazaridou, M.; Christodoulou, E.; Nerantzaki, M.; Kostoglou, M.; Lambropoulou, D.A.; Katsarou, A.; Pantopoulos, K.; Bikiaris, D.N. Formulation and In-Vitro Characterization of Chitosan-Nanoparticles Loaded with the Iron Chelator Deferoxamine Mesylate (DFO). Pharmaceutics 2020, 12, 238. [Google Scholar] [CrossRef] [Green Version]
- Bahrami, H.; Tabrizchi, M.; Farrokhpour, H. Protonation of caffeine: A theoretical and experimental study. Chem. Phys. 2013, 415, 222–227. [Google Scholar] [CrossRef]
- Janes, K.A.; Fresneau, M.P.; Marazuela, A.; Fabra, A.; Alonso, M.J. Chitosan nanoparticles as delivery systems for doxorubicin. J. Control. Release 2001, 73, 255–267. [Google Scholar] [CrossRef]
- Chorilli, M.; Calixto, G.; Rimério, T.C.; Scarpa, M.V. Caffeine Encapsulated in Small Unilamellar Liposomes: Characerization and In Vitro Release Profile. J. Dispers. Sci. Technol. 2013, 34, 1465–1470. [Google Scholar] [CrossRef]
- Ascenso, A.; Batista, C.; Cardoso, P.; Mendes, T.; Praça, F.G.; Bentley, M.V.L.B.; Raposo, S.; Simões, S. Development, characterization, and skin delivery studies of related ultradeformable vesicles: Transfersomes, ethosomes, and transethosomes. Int. J. Nanomed. 2015, 10, 5837–5851. [Google Scholar] [CrossRef] [Green Version]
- Fan, W.; Yan, W.; Xu, Z.; Ni, H. Formation mechanism of monodisperse, low molecular weight chitosan nanoparticles by ionic gelation technique. Colloids Surf. B Biointerfaces 2012, 90, 21–27. [Google Scholar] [CrossRef]
- Hu, B.; Pan, C.; Sun, Y.; Hou, Z.; Ye, H.; Zeng, X. Optimization of Fabrication Parameters To Produce Chitosan−Tripolyphosphate Nanoparticles for Delivery of Tea Catechins. J. Agric. Food Chem. 2008, 56, 7451–7458. [Google Scholar] [CrossRef]
- Papadimitriou, S.; Bikiaris, D.; Avgoustakis, K.; Karavas, E.; Georgarakis, M. Chitosan nanoparticles loaded with dorzolamide and pramipexole. Carbohydr. Polym. 2008, 73, 44–54. [Google Scholar] [CrossRef]
- Aziz, S.N.; Badawy, A.A.; Nessem, D.I.; El Malak, N.S.A. Promising nanoparticulate system for topical delivery of diphenhydramine hydrochloride: In-vitro and in-vivo evaluation. J. Drug Deliv. Sci. Technol. 2020, 55, 101454. [Google Scholar] [CrossRef]
- Leelapornpisid, P.; Leesawat, P.; Natakarnkitkul, S.; Rattanapanadda, P. Application of chitosan for preparation of arbutin nanoparticles as skin whitening. J. Met. Mater. Miner. 2010, 20, 101–105. [Google Scholar]
- Perinelli, D.R.; Campana, R.; Skouras, A.; Bonacucina, G.; Cespi, M.; Mastrotto, F.; Baffone, W.; Casettari, L. Chitosan Loaded into a Hydrogel Delivery System as a Strategy to Treat Vaginal Co-Infection. Pharmaceutics 2018, 10, 23. [Google Scholar] [CrossRef] [Green Version]
- Nobbmann, U. Polydispersity–What Does It Mean for DLS and Chromatography. Available online: http://www.materials-talks.com/blog/2014/10/23/polydispersity-whatdoes-it-mean-for-dls-and-chromatography (accessed on 4 December 2014).
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Davarani, F.H.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [Green Version]
- Shah, B.; Khunt, D.; Bhatt, H.; Misra, M.; Padh, H. Application of quality by design approach for intranasal delivery of rivastigmine loaded solid lipid nanoparticles: Effect on formulation and characterization parameters. Eur. J. Pharm. Sci. 2015, 78, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Bihari, P.; Vippola, M.; Schultes, S.; Praetner, M.; Khandoga, A.G.; A Reichel, C.; Coester, C.; Tuomi, T.; Rehberg, M.; Krombach, F. Optimized dispersion of nanoparticles for biological in vitro and in vivo studies. Part. Fibre Toxicol. 2008, 5, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antoniou, J.; Liu, F.; Majeed, H.; Qi, J.; Yokoyama, W.; Zhong, F. Physicochemical and morphological properties of size-controlled chitosan–tripolyphosphate nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2015, 465, 137–146. [Google Scholar] [CrossRef]
- Hsieh, F.-M.; Huang, C.; Lin, T.-F.; Chen, Y.-M.; Lin, J.-C. Study of sodium tripolyphosphate-crosslinked chitosan beads entrapped with Pseudomonas putida for phenol degradation. Process. Biochem. 2008, 43, 83–92. [Google Scholar] [CrossRef]
- Honary, S.; Zahir, F. Effect of zeta potential on the properties of nano-drug delivery systems-a review (Part 1). Trop. J. Pharm. Res. 2013, 12, 255–264. [Google Scholar]
- Arafa, M.G.; Girgis, G.N.S.; El-Dahan, M.S. Chitosan-Coated PLGA Nanoparticles for Enhanced Ocular Anti-Inflammatory Efficacy of Atorvastatin Calcium. Int. J. Nanomed. 2020, 15, 1335–1347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, J.H.; Cho, M.A.; Son, H.H.; Lee, C.K.; Yoon, M.S.; Cho, H.H.; Seo, D.S.; Kim, K.J. Characterization and Formation of Phospholipid Nanoemulsion Coatings on Mg-Modified Sericite Surface. J. Ind. Eng. Chem. 2006, 12, 635–638. [Google Scholar]
- Campbell, R.B. Positively-charged liposomes for targeting tumor vasculature. In Nanotechnology for Cancer Therapy; CRC Press: Boca Raton, FL, USA, 2006; pp. 620–634. [Google Scholar]
- Gan, Q.; Wang, T.; Cochrane, C.; McCarron, P. Modulation of surface charge, particle size and morphological properties of chitosan–TPP nanoparticles intended for gene delivery. Colloids Surf. B Biointerfaces 2005, 44, 65–73. [Google Scholar] [CrossRef]
- Stoica, R.; Şomoghi, R.; Ion, R. Preparation of chitosan-tripolyphosphate nanoparticles for the encapsulation of polyphenols extracted from rose hips. Dig. J. Nanomater. Biostruct. 2013, 8, 955–963. [Google Scholar]
- Pooja, D.; Bikkina, D.J.B.; Kulhari, H.; Nikhila, N.; Chinde, S.; Raghavendra, Y.; Sreedhar, B.; Tiwari, A.K. Fabrication, characterization and bioevaluation of silibinin loaded chitosan nanoparticles. Int. J. Biol. Macromol. 2014, 69, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Rázga, F.; Vnuková, D.; Némethová, V.; Mazancová, P.; Lacík, I. Preparation of chitosan-TPP sub-micron particles: Critical evaluation and derived recommendations. Carbohydr. Polym. 2016, 151, 488–499. [Google Scholar] [CrossRef]
- Keawchaoon, L.; Yoksan, R. Preparation, characterization and in vitro release study of carvacrol-loaded chitosan nanoparticles. Colloids Surf. B Biointerfaces 2011, 84, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Yab, Z.; Mehdi, S.H.; Ahmad, I.; Rizvi, M.M.A. Characterization and anti-proliferative activity of curcumin loaded chitosan nanoparticles in cervical cancer. Int. J. Biol. Macromol. 2016, 93, 242–253. [Google Scholar] [CrossRef] [PubMed]






| Factors (Independent Variables) | Levels | ||
|---|---|---|---|
| Low (−1) | Medium (0) | High (+1) | |
| 0.05 | 0.15 | 0.25 |
| 3 | 4 | 5 |
| 2:1 | 4:1 | 6:1 |
| Factor Levels in Their Actual Value | |||
|---|---|---|---|
| Formulations | CS Concentration (%) | pH | Mass Ratio (CS:TPP) |
| F1 | 0.05 | 3 | 4:1 |
| F2 | 0.05 | 4 | 2:1 |
| F3 | 0.05 | 5 | 4:1 |
| F4 | 0.05 | 4 | 6:1 |
| F5 | 0.15 | 4 | 4:1 |
| F6 | 0.15 | 3 | 6:1 |
| F7 | 0.15 | 4 | 4:1 |
| F8 | 0.15 | 5 | 6:1 |
| F9 | 0.15 | 3 | 2:1 |
| F10 | 0.15 | 5 | 2:1 |
| F11 | 0.15 | 4 | 4:1 |
| F12 | 0.25 | 3 | 4:1 |
| F13 | 0.25 | 4 | 2:1 |
| F14 | 0.25 | 5 | 4:1 |
| F15 | 0.25 | 4 | 6:1 |
| Formulations | EE ± SD (%) (Y1) | PS ± SD (nm) (Y2) | PDI ± SD (Y3) | ZP ± SD (mV) (Y4) |
|---|---|---|---|---|
| F1 | 7.09 ± 1.81 | 120.2 ± 2.01 | 0.249 ± 0.004 | 39.5 ± 4.11 |
| F2 | 10.13 ± 0.91 | 181.003 ± 8.54 | 0.24 ± 0.008 | 26.0 ± 4.00 |
| F3 | 5.19 ± 1.03 | 163.33 ± 7.64 | 0.261 ± 0.004 | 23.3 ± 1.15 |
| F4 | 3.45 ± 0.52 | 95.33 ± 5.03 | 0.263 ± 0.004 | 27.0 ± 1.00 |
| F5 | 17.22 ± 0.24 | 182.00 ± 3.00 | 0.287 ± 0.003 | 29.5 ± 5.07 |
| F6 | 13.8 ± 0.52 | 121.33 ± 9.07 | 0.273 ± 0.004 | 44.6 ± 0.51 |
| F7 | 16.62 ± 0.29 | 226.00 ± 10.15 | 0.281 ± 0.004 | 32.0 ± 2.00 |
| F8 | 11.11 ± 0.24 | 175.67 ± 5.86 | 0.459 ± 0.005 | 24.7 ± 3.06 |
| F9 | 19.82 ± 0.59 | 323.67 ± 7.77 | 0.392 ± 0.009 | 34.6 ± 4.13 |
| F10 | 17.92 ± 0.41 | 496.00 ± 7.55 | 0.517 ± 0.031 | 22.0 ± 2.00 |
| F11 | 14.06 ± 0.43 | 184.00 ± 6.00 | 0.245 ± 0.005 | 27.3 ± 1.15 |
| F12 | 23.02 ± 0.24 | 463.67 ± 12.66 | 0.473 ± 0.028 | 43.7 ± 1.53 |
| F13 | 26.34 ± 0.52 | 883.67 ± 7.37 | 0.736 ± 0.016 | 27.0 ± 3.00 |
| F14 | 21.15 ± 0.19 | 725.67 ± 6.11 | 0.753 ± 0.004 | 24.0 ± 2.00 |
| F15 | 19.25 ± 0.1 | 345.67 ± 8.51 | 0.553 ± 0.039 | 37.3 ± 2.52 |
| Model | R2 | R2 Adjusted | R2 Predicted | Adequate Precision | Remarks |
|---|---|---|---|---|---|
| Entrapment efficiency percent (Y1) | |||||
| Linear | 0.9814 | 0.9763 | 0.9705 | 43.125 | Suggested |
| 2FI | 0.9814 | 0.9674 | 0.9445 | 27.801 | - |
| Quadratic | 0.9923 | 0.9793 | 0.9833 | 29.158 | - |
| Particle size (Y2) | |||||
| Linear | 0.8155 | 0.7652 | 0.7652 | 12.766 | - |
| 2FI | 0.9013 | 0.8272 | 0.8272 | 11.249 | - |
| Quadratic | 0.9954 | 0.9872 | 0.9872 | 35.272 | Suggested |
| Polydispersity index (Y3) | |||||
| Linear | 0.7834 | 0.7244 | 0.6492 | 11.002 | - |
| 2FI | 0.8499 | 0.7374 | 0.6757 | 8.52 | - |
| Quadratic | 0.996 | 0.9888 | 0.9684 | 34.508 | Suggested |
| Zeta Potential (Y4) | |||||
| Linear | 0.8913 | 0.8617 | 0.7871 | 16.182 | Suggested |
| 2FI | 0.9405 | 0.8959 | 0.7666 | 14.098 | - |
| Quadratic | 0.9768 | 0.9351 | 0.8235 | 14.94 | - |
| Response | Predicted Value | Observed Value | Low Confidence Interval | High Confidence Interval |
|---|---|---|---|---|
| EE% | 16.8412 | 17.25 ± 1.48 | 15.68 | 18.00 |
| PS (nm) | 177.267 | 173.03 ± 4.32 | 128.43 | 226.11 |
| PDI | 0.303 | 0.278 ± 0.01 | 0.27 | 0.34 |
| ZP (mV) | 42.067 | 41.7 ± 3 | 38.96 | 45.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abosabaa, S.A.; ElMeshad, A.N.; Arafa, M.G. Chitosan Nanocarrier Entrapping Hydrophilic Drugs as Advanced Polymeric System for Dual Pharmaceutical and Cosmeceutical Application: A Comprehensive Analysis Using Box-Behnken Design. Polymers 2021, 13, 677. https://doi.org/10.3390/polym13050677
Abosabaa SA, ElMeshad AN, Arafa MG. Chitosan Nanocarrier Entrapping Hydrophilic Drugs as Advanced Polymeric System for Dual Pharmaceutical and Cosmeceutical Application: A Comprehensive Analysis Using Box-Behnken Design. Polymers. 2021; 13(5):677. https://doi.org/10.3390/polym13050677
Chicago/Turabian StyleAbosabaa, Sara A., Aliaa N. ElMeshad, and Mona G. Arafa. 2021. "Chitosan Nanocarrier Entrapping Hydrophilic Drugs as Advanced Polymeric System for Dual Pharmaceutical and Cosmeceutical Application: A Comprehensive Analysis Using Box-Behnken Design" Polymers 13, no. 5: 677. https://doi.org/10.3390/polym13050677