Recovered Energy from Salinity Gradients Utilizing Various Poly(Acrylic Acid)-Based Hydrogels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of PAA Gels, IPN PAA/PVSA Gels and Semi-IPN PAA/PSSA-MA Gels
2.3. Salinity Gradient Setup and Operation
2.4. Swelling Ratio
2.5. Oscillatory Shear Rheology
2.6. Structure Characterization
2.7. Recovered Energy
2.8. Energy Efficiency
3. Results and Discussion
3.1. Swelling Ratio of Hydrogels
3.2. Rheological Properties of the Hydrogels
3.3. FTIR Spectrum
3.4. Swelling Volume with Multi-Cycle Measurements
3.5. Volume Expansion Rate
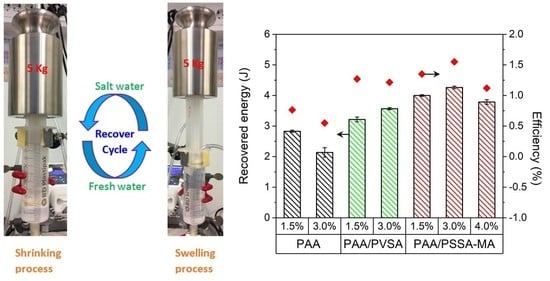
3.6. Recovered Energy and Energy Efficiency
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Panwar, N.L.; Kaushik, S.C.; Kothari, S. Role of renewable energy sources in environmental protection: A review. Renew. Sustain. Energy Rev. 2011, 15, 1513–1524. [Google Scholar] [CrossRef]
- Galán-Martín, A.; Pozo, C.; Azapagic, A.; Grossmann, I.E.; Dowelld, N.M.; Guillén-Gosálbez, G. Time for global action: An optimised cooperative approach towards effective climate change mitigation. Energy Environ. Sci. 2018, 11, 572–581. [Google Scholar] [CrossRef] [Green Version]
- Rockström, J.; Gaffney, O.; Rogelj, J.; Meinshausen, M.; Nakicenovic, N.; Schellnhuber, H.J. A roadmap for rapid decarbonization. Science 2017, 355, 1269–1271. [Google Scholar]
- Yip, N.Y.; Brogioli, D.; Hamelers, H.V.M.; Nijmeijer, K. Salinity Gradients for Sustainable Energy: Primer, Progress, and Prospects. Environ. Sci. Technol. 2016, 50, 12072–12094. [Google Scholar] [CrossRef]
- Chu, S.; Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Dresselhaus, M.S.; Thomas, I.L. Alternative energy technologies. Nature 2001, 414, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Hoffert, M.I.; Caldeira, K.; Benford, G.; Criswell, D.R.; Green, C.; Herzog, H.; Jain, A.K.; Kheshgi, H.S.; Lackner, K.S.; Lewis, J.S.; et al. Advanced Technology Paths to Global Climate Stability: Energy for a Greenhouse Planet. Science 2002, 298, 981–987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norman, R.S. Water salination: A source of energy. Science 1974, 186, 350–352. [Google Scholar] [CrossRef]
- Pattle, R.E. Production of electric power by mixing fresh and salt water in the hydroelectric pile. Nature 1954, 174, 660. [Google Scholar] [CrossRef]
- Straub, A.P.; Deshmukh, A.; Elimelech, M. Pressure-retarded osmosis for power generation from salinity gradients: Is it viable? Energy Environ. Sci. 2015, 9, 31–48. [Google Scholar] [CrossRef]
- Ramon, G.Z.; Feinberg, B.J.; Hoek, E.M.V. Membrane-based production of salinity-gradient power. Energy Environ. Sci. 2011, 4, 4423–4434. [Google Scholar] [CrossRef]
- Isaacs, J.D.; Seymour, R.J. The ocean as a power resource. Int. J. Environ. Stud. 1973, 4, 201–205. [Google Scholar] [CrossRef]
- Logan, B.E.; Elimelech, M. Membrane-based processes for sustainable power generation using water. Nature 2012, 488, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Sarp, S.; Li, Z.; Saththasivam, J. Pressure Retarded Osmosis (PRO): Past experiences, current developments, and future prospects. Desalination 2016, 389, 2–14. [Google Scholar] [CrossRef]
- Levenspiel, O.B.; de Nevers, N. The osmotic pump. Science 1974, 183, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Achilli, A.; Childress, A.E. Pressure retarted osmosis: From the vision of Sidney Loeb to the first prototype installation-Review. Desalination 2010, 261, 205–211. [Google Scholar] [CrossRef]
- Feinberg, B.J.; Ramon, G.Z.; Hoek, E.M. Thermodynamic analysis of osmotic energy recovery at a reverse osmosis desalination plant. Environ. Sci. Technol. 2013, 47, 2982–2989. [Google Scholar] [CrossRef] [PubMed]
- Post, J.W.; Hamalers, H.V.M.; Buisman, C.J.N. Energy recovery from controlled mixing salt and fresh water with a reverse electrodialysis system. Environ. Sci. Technol. 2008, 42, 5785–5790. [Google Scholar] [CrossRef]
- Yip, N.Y.; Vermaas, D.A.; Nijmeijer, K.; Elimelech, M. Thermodynamic, energy efficiency, and power density analysis of reverse electrodialysis power generation with natural salinity gradient. Environ. Sci. Technol. 2014, 48, 4925–4936. [Google Scholar] [CrossRef] [PubMed]
- Veerman, J.; Saakes, M.; Metz, S.J.; Harmsen, G.J. Reverse electrodialysis: Evaluation of suitable electrode systems. J. Appl. Electrochem. 2010, 40, 1461–1474. [Google Scholar] [CrossRef] [Green Version]
- Long, R.; Kuang, Z.; Liu, Z.; Liu, W. Reverse electrodialysis in bilayer nanochannels: Salinity gradient-driven power generation. Phys. Chem. Chem. Phys. 2018, 20, 7295–7302. [Google Scholar] [CrossRef]
- Moreno, J.; Grasman, S.; van Engelen, R.; Nijmeijer, K. Upscaling Reverse Electrodialysis. Environ. Sci. Technol. 2018, 52, 10856–10863. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Kim, T.; Rahimi, M.; Gorski, C.A.; Logan, B.E. Integrating Reverse-Electrodialysis Stacks with Flow Batteries for Improved Energy Recovery from Salinity Gradients and Energy Storage. ChemSusChem 2017, 10, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Rica, R.; Ziano, R.; Salerno, D.; Mantegazza, F.; van Roij, R.; Brogioli, D. Capacitive mixing for harvesting the free energy of solution at different concentrations. Entropy 2013, 15, 1388–1407. [Google Scholar] [CrossRef] [Green Version]
- Brogioli, D. Extracting renewable energy from a salinity difference using a capacitor. Phys. Rev. Lett. 2009, 103, 058501. [Google Scholar] [CrossRef] [PubMed]
- Sales, B.B.; Liu, F.; Schaetzle, O.; Buisman, C.J.N.; Hamelers, H.V.M. Electrochemical characterization of a supercapacitor flow cell for power production from salinity gradients. Electrochim. Acta 2012, 86, 298–304. [Google Scholar] [CrossRef] [Green Version]
- Bijmans, M.F.M.; Burheim, O.S.; Bryjak, M.; Delgado, A.; Hack, P.; Mantegazza, F.; Tenisson, S.; Hamelers, H.V.M. Capmix-deploying capacitors for salt gradient power extraction. Energy Procedia 2012, 20, 108–115. [Google Scholar] [CrossRef] [Green Version]
- Hatzell, M.C.; Raju, M.; Watson, V.J.; Stack, A.G.; van Duin, A.C.; Logan, B.E. Effect of strong acid functional groups on electrode rise potential in capacitive mixing by double layer expansion. Environ. Sci. Technol. 2014, 48, 14041–14048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, X.; Yang, W.; Hatzell, M.C.; Logan, B.E. Energy Recovery from Solutions with Different Salinities Based on Swelling and Shrinking of Hydrogels. Environ. Sci. Technol. 2014, 48, 7157–7163. [Google Scholar] [CrossRef]
- Arens, L.; Weißenfeld, F.; Klein, C.O.; Schlag, K.; Wilhelm, M. Osmotic Engine: Translating Osmotic Pressure into Macroscopic Mechanical Force via Poly(Acrylic Acid) Based Hydrogels. Adv. Sci. 2017, 4, 1700112. [Google Scholar] [CrossRef] [Green Version]
- Bui, T.Q.; Cao, V.D.; Do, N.B.D.; Christoffersen, T.E.; Wang, W.; Kjøniksen, A.L. Salinity gradient energy from expasion and contraction of poly(allylamine hydrochloride) hydorgels. Acs Appl. Mater. Interfaces 2018, 10, 22218–22225. [Google Scholar] [CrossRef] [PubMed]
- Zavahir, S.; Krupa, I.; Almaadeed, S.A.; Tkac, J.; Kasak, P. Polyzwitterionic hydrogels in engines based on the anipoyelectrolyte effect and driven by the salinity gradient. Environ. Sci. Technol. 2019, 53, 9260–9268. [Google Scholar] [CrossRef]
- Maleki, A.; Kjøniksen, A.; Nyström, B. Characterization of the chemical degradation of hyaluronic acid during chemical gelation in the presence of different cross-linker agents. Carbohydr. Res. 2007, 342, 2776–2792. [Google Scholar] [CrossRef] [PubMed]
- Bui, Q.T.; Jeon, Y.-S.; Um, S.H.; Chung, D.J.; Kim, J.-H. Preparation of novel hybrid gels from polyaspartamides and natural alginate or hyaluronate by click reaction. J. Polym. Res. 2015, 22, 27. [Google Scholar] [CrossRef]
- Singhal, R.; Gupta, K. A Review: Tailor-made Hydrogel Structures(Classifications and Synthesis Parameters). Polym.-Plast. Technol. Eng. 2016, 55, 54–70. [Google Scholar] [CrossRef]
- Roy, S.G.; Haldar, U.; De, P. Remarkable swelling capability of amino acid based cross-linked polymer networks in organic and aqueous medium. Acs Appl. Mater. Interfaces 2014, 6, 4233–4241. [Google Scholar] [CrossRef]
- Sharma, S.; Dua, A.; Malik, A. Polyaspartic acid based superabsorbent polymers. Eur. Polym. J. 2014, 59, 363–376. [Google Scholar] [CrossRef]
- Papadakis, C.M.; Tsitsilianis, C. Responsive Hydrogels from Associative Block Copolymers: Physical Gelling through Polyion Complexation. Gels 2017, 3, 3. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, S.; Hui, P.C.-l.; Wat, E.; Kan, C.-w.; Leung, P.-C.; Wang, W. Drug delivery system of dual-responsive PF127 hydrogel with polysaccharide-based nano-conjugate for textile-based transdermal therapy. Carbohydr. Polym. 2020, 236, 116074. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.S.H.; Ashton, M.; Dodou, K. Effect of Crosslinking Agent Concentration on the Properties of Unmedicated Hydrogels. Pharmaceutics 2015, 7, 305–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, S.; Gu, L. Influence of Crosslink Density and Stiffness on Mechanical Properties of Type I Collagen Gel. Materials 2015, 8, 551–560. [Google Scholar] [CrossRef]
- Perera, D.I.; Shanks, R.A. Swelling and Mechanical Properties of Crosslinked Hydrogels Containing N-Vinyl pyrrol idone. Polym. Int. 1996, 39, 121–127. [Google Scholar] [CrossRef]
- Maleki, A.; Beheshti, N.; Zhu, K.; Kjøniksen, A.; Nyström, B. Shrinking of chemically cross-linked polymer networks in the postgel region. Polym. Bull. 2007, 58, 435–445. [Google Scholar] [CrossRef]
- Chassenieux, C.; Tsitsilianis, C. Recent trends in pH/thermo-responsive self-assembling hydrogels: From polyions to peptide-based polymeric gelators. Soft Matter 2016, 12, 1344–1359. [Google Scholar] [CrossRef] [PubMed]
- Richtering, W.; Saunders, B.R. Gel architectures and their complexity. Soft Matter 2014, 10, 3695–3702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Na, Y.H. Double network hydrogels with extremely high toughness and their applications. Korea-Aust. Rheol. J. 2013, 25, 185–196. [Google Scholar] [CrossRef]
- Shibayama, M. Structure-mechanical property relationship of tough hydrogels. Soft Matter 2012, 8, 8030–8038. [Google Scholar] [CrossRef]
- Gong, J.P. Why are double network hydrogels so tough? Soft Matter. 2010, 6, 2583–2590. [Google Scholar] [CrossRef]
- Zhao, X.H. Multi-scale multi-mechanism design of tough hydrogels: Building dissipation into stretchy networks. Soft Matter. 2014, 10, 672–687. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Wu, Z.; Lai, D.; Zheng, M.; Xu, L.; Huo, J.; Chen, Z.; Yuan, B.; Fu, M.-L. Resilient biomass-derived hydrogel with tailored topography for highly efficient and long-term solar evaporation of high-salinity brine. J. Mater. Chem. A 2020, 8, 22645–22656. [Google Scholar] [CrossRef]
- Guo, Y.; Lu, H.; Zhao, F.; Zhou, X.; Shi, W.; Yu, G. Biomass-Derived Hybrid Hydrogel Evaporators for Cost-Effective Solar Water Purification. Adv. Mater. 2020, 32, 1907061. [Google Scholar] [CrossRef]
- Gao, H.; Mao, J.; Cai, Y.; Li, S.; Fu, Y.; Liu, X.; Liang, H.; Zhao, T.; Liu, M.; Jiang, L. Euryhaline Hydrogel with Constant Swelling and Salinity-Enhanced Mechanical Strength in a Wide Salinity Range. Adv. Funct. Mater. 2021, 31, 2007664. [Google Scholar] [CrossRef]
- Meng, Y.; Ye, L. Synthesis and swelling property of superabsorbent starch grafted with acrylic acid/2-acrylamido-2-methyl-1-propanesulfonic acid. J. Sci. Food Agric. 2017, 97, 3831–3840. [Google Scholar] [CrossRef]
- Wu, M.; Kaur, P.; Yue, H.; Clemmens, A.M.; Waldeck, D.H.; Xue, C.; Liu, H. Charge density effects on the aggregation properties of poly(p-phenylene-ethynylene)-based anionic polyelectrolytes. J. Phys. Chem. B 2008, 112, 3300–3310. [Google Scholar] [CrossRef]
- Mohan, Y.M.; Dickson, J.P.; Geckeler, K.E. Swelling and diffusion characteristics of novel semi-interpenetrating network hydrogels composed of poly[(acrylamide)-co-(sodium acrylate)] and poly[(vinylsulfonic acid), sodium salt]. Polym. Int. 2007, 56, 175–185. [Google Scholar] [CrossRef]
- Hussain, T.; Ansari, M.; Ranjha, N.M.; Khan, I.U.; Shahzad, Y. Chemically Cross-Linked Poly(acrylic-co-vinylsulfonic) Acid Hydrogel for the Delivery of Isosorbide Mononitrate. Sci. World J. 2013, 2013, 340737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nesic, A.; Panic, V.; Ostojic, S.; Micic, D.; Pajic-Lijakovic, I.; Onjia, A.; Velickovic, S. Physical-chemical behavior of novel copolymers composed of methacrylic acid and 2-acrylamido-2-methylpropane sulfonic acid. Mater. Chem. Phys. 2016, 174, 156–163. [Google Scholar] [CrossRef]
- Zuidema, J.M.; Rivet, C.J.; Gilbert, R.J.; Morrison, F.A. A protocol for rheological characterization of hydrogels for tissue engineering strategies. J. Biomed. Mater. Res. Part B: Appl. Biomater. 2014, 102, 1063–1073. [Google Scholar] [CrossRef]
- Todica, M.; Stefan, R.; Pop, C.V.; Olar, L. IR and Raman investigation of some poly(acrylic) acid gels inaqueous and neutralize state. Acta Phys. Pol. A 2015, 128, 128–135. [Google Scholar] [CrossRef]
- Atta, A.M.; El Wahab, Z.H.A.; El Shafey, Z.A.; Zidan, W.I.; Akl, Z.F. Characterization and Evaluation of Acrylic Acid Co-2-acrylamido-2-methylpropane-1-sulfonic Acid Hydrogels for Uranium Recovery. J. Dispers. Sci. Technol. 2010, 31, 1415–1422. [Google Scholar] [CrossRef]
- Hoerter, M.; Opera, A.; Barsan, N.; Weimar, U. Chemical interaction of gaseous ammonia and water vapour with polyacrylic acid layers. Sens. Actuators B Chem. 2008, 134, 743–749. [Google Scholar] [CrossRef]
- Dong, J.; Ozaki, Y.; Nakashima, K. FTIR studies of conformational energies of poly(acrylic acid) in cast films. J. Polym. Sci. Part B Polym. Phys. 1997, 35, 507–515. [Google Scholar] [CrossRef]
- Sand, A.; Yadav, M.; Behari, K. Preparation and characterization of modified sodium carboxymethyl cellulose via free radical graft copolymerization of vinyl sulfonic acid in aqueous media. Carbohydr. Polym. 2010, 81, 97–103. [Google Scholar] [CrossRef]
- Nandi, S.; Winter, H.H. Swelling Behavior of Partially Cross-Linked Polymers: A Ternary System. Macromolecules 2005, 38, 4447–4455. [Google Scholar] [CrossRef] [Green Version]
- Bin Imran, A.; Seki, T.; Takeoka, Y. Recent advances in hydrogels in terms of fast stimuli responsiveness and superior mechanical performance. Polym. J. 2010, 42, 839–851. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zhao, C.; Xiang, N.; Li, W. Chain engtanglements and hydrogen bonds in Carbopol microgel reinforced hydrogel. Macromol. Chem. Phys. 2016, 217, 2139–2144. [Google Scholar] [CrossRef]
- Üzüm, Ö.B.; Çetin, G.; Kundakcı, S.; Karadağ, E. Swelling and dye adsorption properties of polyelectrolyte semi-IPNs including of acrylamide/(3-acrylamidopropyl)trimethyl ammonium chloride/poly(ethylene glycol). Sep. Sci. Technol. 2020, 55, 3307–3319. [Google Scholar] [CrossRef]











| Sample Denotation | AA (g) | VSA (mL) | PSSA-MA (g) | MBA (g) | APS (g) | TEMED (mL) | Gelation Time (min) |
|---|---|---|---|---|---|---|---|
| PAA1.5 | 2.5 | - | - | 0.08 | 0.04 | 0.24 | 120 |
| PAA3.0 | 2.5 | - | - | 0.16 | 0.04 | 0.24 | 40 |
| PAA/PVSA1.5 | 2.5 | 4 | - | 0.10 | 0.04 | 0.24 | 120 |
| PAA/PVSA3.0 | 2.5 | 4 | - | 0.20 | 0.04 | 0.24 | 60 |
| PAA/PSSA-MA1.5 | 2.5 | - | 1 | 0.08 | 0.04 | 0.24 | 120 |
| PAA/PSSA-MA3.0 | 2.5 | - | 1 | 0.16 | 0.04 | 0.24 | 60 |
| PAA/PSSA-MA4.0 | 2.5 | - | 1 | 0.21 | 0.04 | 0.24 | 40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bui, T.Q.; Cao, V.D.; Wang, W.; Kjøniksen, A.-L. Recovered Energy from Salinity Gradients Utilizing Various Poly(Acrylic Acid)-Based Hydrogels. Polymers 2021, 13, 645. https://doi.org/10.3390/polym13040645
Bui TQ, Cao VD, Wang W, Kjøniksen A-L. Recovered Energy from Salinity Gradients Utilizing Various Poly(Acrylic Acid)-Based Hydrogels. Polymers. 2021; 13(4):645. https://doi.org/10.3390/polym13040645
Chicago/Turabian StyleBui, Tri Quang, Vinh Duy Cao, Wei Wang, and Anna-Lena Kjøniksen. 2021. "Recovered Energy from Salinity Gradients Utilizing Various Poly(Acrylic Acid)-Based Hydrogels" Polymers 13, no. 4: 645. https://doi.org/10.3390/polym13040645