The Role of Biopolymer-Based Materials in Obstetrics and Gynecology Applications: A Review
Abstract
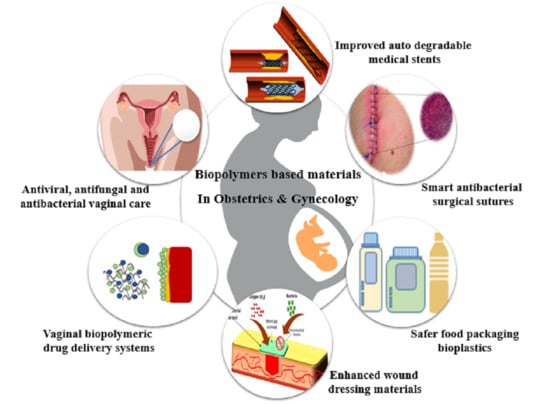
:1. Introduction
2. Health Impact of Conventional Polymer-Based Materials in Obstetrics and Gynecology
2.1. Cosmetics and Personal Care Materials
2.2. Therapeutic Pharmaceuticals
2.3. Surgical Sutures
2.4. Other Applications
3. Biopolymer-Based Materials in Obstetrics and Gynecological Applications
3.1. Biopolymer-Based Materials in Cosmetics and Personal Care
3.2. Biopolymer-Based Materials in Obstetrics and Gynecological Therapeutics
3.3. Biopolymer-Based Materials in Obstetrics and Gynecological Surgical Sutures
3.4. Biopolymer-Based Materials for Obstetrical and Gynecological Wound Management
4. Issues and Challenges of Biopolymers in Obstetrics and Gynecology
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- HPS, A.K.; Saurabh, C.K.; Adnan, A.S.; Fazita, M.N.; Syakir, M.I.; Davoudpour, Y.; Rafatullah, M.; Abdullah, C.K.; Haafiz, M.K.M.; Dungani, R. A review on chitosan-cellulose blends and nanocellulose reinforced chitosan biocomposites: Properties and their applications. Carbohydr. Polym. 2016, 150, 216–226. [Google Scholar]
- Iftikhar, P.M.; Kuijpers, M.V.; Khayyat, A.; Iftikhar, A.; De Sa, M.D. Artificial Intelligence: A New Paradigm in Obstetrics and Gynecology Research and Clinical Practice. Cureus 2020, 12, e7124. [Google Scholar] [CrossRef] [Green Version]
- Racicot, K.; Kwon, J.-Y.; Aldo, P.; Silasi, M.; Mor, G. Understanding the Complexity of the Immune System during Pregnancy. Am. J. Reprod. Immunol. 2014, 72, 107–116. [Google Scholar] [CrossRef]
- Tcheremenskaia, O.; Battistelli, C.L.; Giuliani, A.; Benigni, R.; Bossa, C. In silico approaches for prediction of genotoxic and carcinogenic potential of cosmetic ingredients. Comput. Toxicol. 2019, 11, 91–100. [Google Scholar] [CrossRef]
- Flaviano, M.; Harville, E.W. Adverse Childhood Experiences on Reproductive Plans and Adolescent Pregnancy in the Gulf Resilience on Women’s Health Cohort. Int. J. Environ. Res. Public Health 2020, 18, 165. [Google Scholar] [CrossRef]
- Liao, K.-W.; Kuo, P.-L.; Huang, H.-B.; Chang, J.-W.; Chiang, H.-C.; Huang, P.-C. Increased risk of phthalates exposure for recurrent pregnancy loss in reproductive-aged women. Environ. Pollut. 2018, 241, 969–977. [Google Scholar] [CrossRef]
- Biondi, O.; Motta, S.; Mosesso, P. Low molecular weight polyethylene glycol induces chromosome aberrations in Chinese hamster cells cultured in vitro. Mutagenesis 2002, 17, 261–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yahya, E.B.; Alqadhi, A.M. Recent trends in cancer therapy: A review on the current state of gene delivery. Life Sci. 2021, 269, 119087. [Google Scholar] [CrossRef]
- Perez-Puyana, V.; Jiménez-Rosado, M.; Romero, A.; Guerrero, A. Polymer-Based Scaffolds for Soft-Tissue Engineering. Polymers 2020, 12, 1566. [Google Scholar] [CrossRef]
- Olaiya, N.G.; Nuryawan, A.; Oke, P.K.; Khalil, H.P.S.A.; Rizal, S.; Mogaji, P.B.; Sadiku, E.R.; Suprakas, S.R.; Farayibi, P.K.; Ojijo, V.; et al. The Role of Two-Step Blending in the Properties of Starch/Chitin/Polylactic Acid Biodegradable Composites for Biomedical Applications. Polymers 2020, 12, 592. [Google Scholar] [CrossRef] [Green Version]
- Puri, V.; Sharma, A.; Kumar, P.; Singh, I. Thiolation of Biopolymers for Developing Drug Delivery Systems with Enhanced Mechanical and Mucoadhesive Properties: A Review. Polymers 2020, 12, 1803. [Google Scholar] [CrossRef] [PubMed]
- Teepoo, S.; Dawan, P.; Barnthip, N. Electrospun Chitosan-Gelatin Biopolymer Composite Nanofibers for Horseradish Peroxidase Immobilization in a Hydrogen Peroxide Biosensor. Biosensors 2017, 7, 47. [Google Scholar] [CrossRef] [Green Version]
- Tamer, T.M.; Collins, M.N.; Valachová, K.; Hassan, M.A.; Omer, A.M.; Mohy-Eldin, M.S.; Švík, K.; Jurčík, R.; Ondruška, L.; Biró, C.; et al. MitoQ Loaded Chitosan-Hyaluronan Composite Membranes for Wound Healing. Materials 2018, 11, 569. [Google Scholar] [CrossRef] [Green Version]
- Park, S.-B.; Lih, E.; Park, K.-S.; Joung, Y.K.; Han, D.K. Biopolymer-based functional composites for medical applications. Prog. Polym. Sci. 2017, 68, 77–105. [Google Scholar] [CrossRef]
- Rizal, S.; Olaiya, F.; Saharudin, N.; Abdullah, C.; Olaiya, N.G.; Haafiz, M.M.; Yahya, E.; Sabaruddin, F.; Ikramullah; Abdul Khalil, H.P.S. Isolation of Textile Waste Cellulose Nanofibrillated Fibre Reinforced in Polylactic Acid-Chitin Biodegradable Composite for Green Packaging Application. Polymers 2021, 13, 325. [Google Scholar] [CrossRef]
- Khalil, H.A.; Adnan, A.; Yahya, E.B.; Olaiya, N.; Safrida, S.; Hossain, S.; Balakrishnan, V.; Gopakumar, D.A.; Abdullah, C.; Oyekanmi, A.; et al. A Review on Plant Cellulose Nanofibre-Based Aerogels for Biomedical Applications. Polymers 2020, 12, 1759. [Google Scholar] [CrossRef]
- Shehata, N.; Hassounah, I.; Sobolciak, P.; Krupa, I.; Lewis, R.; Kandas, I. Spider silk fibers: Synthesis, characterization, and related biomedical applications. In Materials for Biomedical Engineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 289–307. [Google Scholar]
- Moohan, J.; Stewart, S.A.; Espinosa, E.; Rosal, A.; Rodríguez, A.; Larrañeta, E.; Donnelly, R.F.; Domínguez-Robles, J. Cellulose Nanofibers and Other Biopolymers for Biomedical Applications. A Review. Appl. Sci. 2019, 10, 65. [Google Scholar] [CrossRef] [Green Version]
- Yahya, E.B.; Jummaat, F.; Amirul, A.A.; Adnan, A.S.; Olaiya, N.G.; Abdullah, C.K.; Rizal, S.; Haafiz, M.K.M.; Khalil, H.P.S.A. A Review on Revolutionary Natural Biopolymer-Based Aerogels for Antibacterial Delivery. Antibiotics 2020, 9, 648. [Google Scholar] [CrossRef] [PubMed]
- Dahy, H. Biocomposite materials based on annual natural fibres and biopolymers—Design, fabrication and customized applications in architecture. Constr. Build. Mater. 2017, 147, 212–220. [Google Scholar] [CrossRef]
- Sumrith, N.; Rangappa, S.M.; Dangtungee, R.; Siengchin, S.; Jawaid, M.; Pruncu, C.I. Biopolymers-Based Nanocomposites: Properties and Applications. In Bio-Based Polymers and Nanocomposites; Springer: Berlin/Heidelberg, Germany, 2019; pp. 255–272. [Google Scholar] [CrossRef]
- Stanisław, M.; Alina, S.; Amit, J. Biopolymers for hydrogels in cosmetics. J. Mater. Sci. Mater. Med. 2020, 31. [Google Scholar] [CrossRef]
- Parveen, I.; Mahmud, I.; Khan, R.A. Biodegradable Natural Polymers for Biomedical Applications. Sci. Rev. 2019, 67–80. [Google Scholar] [CrossRef]
- Huang, H.-M. Medical Application of Polymer-Based Composites. Polymers 2020, 12, 2560. [Google Scholar] [CrossRef] [PubMed]
- Romero, M.; Macchione, M.A.; Mattea, F.; Strumia, M. The role of polymers in analytical medical applications. A review. Microchem. J. 2020, 159, 105366. [Google Scholar] [CrossRef]
- Rizal, S.; Lai, T.K.; Muksin, U.; Olaiya, N.G.; Abdullah, C.K.; Ikramullah; Yahya, E.B.; Chong, E.W.N.; Khalil, H.P.S.A. Properties of Macroalgae Biopolymer Films Reinforcement with Polysaccharide Microfibre. Polymers 2020, 12, 2554. [Google Scholar] [CrossRef]
- Pritchard, N.; Kaitu’U-Lino, T.; Harris, L.; Tong, S.; Hannan, N. Nanoparticles in pregnancy: The next frontier in reproductive therapeutics. Hum. Reprod. Updat. 2020. [Google Scholar] [CrossRef]
- Vizcarra, D.A.M.; Silva, Y.P.; Bruno, J.B.; Brito, D.C.C.; Berrocal, D.D.; Silva, L.M.; Morais, M.L.G.D.S.; Alves, B.G.; Alves, K.A.; Cibin, F.W.S.; et al. Use of synthetic polymers improves the quality of vitrified caprine preantral follicles in the ovarian tissue. Acta Histochem. 2020, 122, 151484. [Google Scholar] [CrossRef]
- Lambert, M.R.; Edwards, T.M. Hormonally active phytochemicals and vertebrate evolution. Evol. Appl. 2017, 10, 419–432. [Google Scholar] [CrossRef] [PubMed]
- Kaji, T. Genitourinary Tract Abnormalities. In Fetal Morph Functional Diagnosis; Springer: Berlin/Heidelberg, Germany, 2020; pp. 137–148. [Google Scholar]
- Chung, J.H.-Y.; Simmons, A.; Poole-Warren, L.A. Non-degradable polymer nanocomposites for drug delivery. Expert Opin. Drug Deliv. 2011, 8, 765–778. [Google Scholar] [CrossRef] [PubMed]
- Major, I.; Fuenmayor, E.; McConville, C. The Production of Solid Dosage Forms from Non-Degradable Polymers. Curr. Pharm. Des. 2016, 22, 2738–2760. [Google Scholar] [CrossRef]
- Akceylan, E.; Bahadir, M.; Yılmaz, M. Removal efficiency of a calix[4]arene-based polymer for water-soluble carcinogenic direct azo dyes and aromatic amines. J. Hazard. Mater. 2009, 162, 960–966. [Google Scholar] [CrossRef]
- Oppenheimer, B.S.; Oppenheimer, E.T.; Danishefsky, I.; Stout, A.P.; Eirich, F.R. Further studies of polymers as carcinogenic agents in animals. Cancer Res. 1955, 15, 333–340. [Google Scholar]
- Combes, R.; Haveland-Smith, R. A review of the genotoxicity of food, drug and cosmetic colours and other azo, triphenylmethane and xanthene dyes. Mutat. Res. Genet. Toxicol. 1982, 98, 101–243. [Google Scholar] [CrossRef]
- Quan, W.-Y.; Kong, S.-Z.; Li, S.-D.; Liu, H.-Z.; Ouyang, Q.-Q.; Huang, Y.-M.; Luo, H. Grafting of 18β-Glycyrrhetinic Acid and Sialic Acid onto Chitosan to Produce a New Amphipathic Chitosan Derivative: Synthesis, Characterization, and Cytotoxicity. Molecules 2021, 26, 452. [Google Scholar] [CrossRef]
- Khadivi, P.; Salami-Kalajahi, M.; Roghani-Mamaqani, H.; Sofla, R.L.M. Polydimethylsiloxane-based Polyurethane/cellulose Nanocrystal Nanocomposites: From Structural Properties Toward Cytotoxicity. Silicon 2021, 1–9. [Google Scholar] [CrossRef]
- Diemer, F.; Stark, H.; Helfgen, E.-H.; Enkling, N.; Probstmeier, R.; Winter, J.; Kraus, D. In vitro cytotoxicity of different dental resin-cements on human cell lines. J. Mater. Sci. Mater. Med. 2021, 32, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Çobanoğlu, H.; Belivermiş, M.; Sıkdokur, E.; Kılıç, Ö.; Çayır, A. Genotoxic and cytotoxic effects of polyethylene microplastics on human peripheral blood lymphocytes. Chemosphere 2021, 272, 129805. [Google Scholar] [CrossRef]
- Nohynek, G.; Dufour, E.; Roberts, M. Nanotechnology, Cosmetics and the Skin: Is There a Health Risk? Skin Pharmacol. Physiol. 2008, 21, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Kraus, T.A.; Engel, S.M.; Sperling, R.S.; Kellerman, L.; Lo, Y.; Wallenstein, S.; Escribese, M.M.; Garrido, J.L.; Singh, T.; Loubeau, M.; et al. Characterizing the Pregnancy Immune Phenotype: Results of the Viral Immunity and Pregnancy (VIP) Study. J. Clin. Immunol. 2011, 32, 300–311. [Google Scholar] [CrossRef]
- Amstutz, H.C.; Campbell, P.; Kossovsky, N.; Clarke, I.C. Mechanism and clinical significance of wear debris-induced osteolysis. Clin. Orthop. Relat. Res. 1992, 276, 7–18. [Google Scholar] [CrossRef]
- Hirakawa, K.; Bauer, T.W.; Stulberg, B.N.; Wilde, A.H. Comparison and quantitation of wear debris of failed total hip and total knee arthroplasty. J. Biomed. Mater. Res. 1996, 31, 257–263. [Google Scholar] [CrossRef]
- Thenmozhi, H.; Rajan, M.M.; Ahmed, K. D-shaped PCF sensor based on SPR for the detection of carcinogenic agents in food and cosmetics. Optik 2019, 180, 264–270. [Google Scholar] [CrossRef]
- Bukhari, N.; Joseph, J.P.; Hussain, S.S.; Adeeb, M.A.M.; Wakim, M.J.Y.; Yahya, E.; Arif, A.; Saleem, A.; Sharif, N.; Khan, M.A. Prevalence of Human Papilloma Virus Sub Genotypes following Head and Neck Squamous Cell Carcinomas in Asian Continent, A Systematic Review Article. Asian Pac. J. Cancer Prev. 2019, 20, 3269–3277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robbins, C.R.; Robbins, C.R. Chemical and Physical Behavior of Human Hair; Springer: Berlin/Heidelberg, Germany, 2012; Volume 4. [Google Scholar]
- Turati, F.; Pelucchi, C.; Galeone, C.; DeCarli, A.; La Vecchia, C. Personal hair dye use and bladder cancer: A meta-analysis. Ann. Epidemiol. 2014, 24, 151–159. [Google Scholar] [CrossRef]
- Schlatter, H.; Long, T.; Gray, J. An overview of hair dye safety. J. Cosmet. Dermatol. 2007, 6, 32–36. [Google Scholar] [CrossRef]
- Fruijtier-Pölloth, C. Safety assessment on polyethylene glycols (PEGs) and their derivatives as used in cosmetic products. Toxicology 2005, 214, 1–38. [Google Scholar] [CrossRef]
- Yahya, E.B.; AbdulSamad, M.A.; Allaq, A.A.; Abdoallah, T.; Ermese, E. The Effect of Natural and Petroleum Based Materials on the Growth Rate and Antibiotic Sensitivity of Pseudomonas aeruginosa. Int. J. Res. Appl. Sci. Biotechnol. 2020, 7, 295–298. [Google Scholar] [CrossRef]
- Suarez-Torres, J.D.; Jimenez-Orozco, F.A.; Ciangherotti, C.E. Drug excipients, food additives, and cosmetic ingredients probably not carcinogenic to humans reveal a functional specificity for the 2-year rodent bioassay. J. Appl. Toxicol. 2020, 40, 1113–1130. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Li, J.; Xu, S.; Zhou, Y.; Zhao, H.; Li, Y.; Xiong, C.; Sun, X.; Liu, H.; Liu, W.; et al. Prenatal exposure to bisphenol A and its alternatives and child neurodevelopment at 2 years. J. Hazard. Mater. 2020, 388, 121774. [Google Scholar] [CrossRef] [PubMed]
- Sugeng, E.J.; Symeonides, C.; O’Hely, M.; Vuillermin, P.; Sly, P.D.; Vijayasarathy, S.; Thompson, K.; Pezic, A.; Mueller, J.F.; Ponsonby, A.-L. Predictors with regard to ingestion, inhalation and dermal absorption of estimated phthalate daily intakes in pregnant women: The Barwon infant study. Environ. Int. 2020, 139, 105700. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.-M.; Dhar, U.; Calafat, A.M.; Nguyen, V.; Schmidt, R.J.; Hertz-Picciotto, I. Temporal Trends of Exposure to Phthalates and Phthalate Alternatives in California Pregnant Women during 2007–2013: Comparison with Other Populations. Environ. Sci. Technol. 2020, 54, 13157–13166. [Google Scholar] [CrossRef]
- Jeong, S.-H.; Jang, J.-H.; Cho, H.-Y.; Lee, Y.-B. Risk assessment for humans using physiologically based pharmacokinetic model of diethyl phthalate and its major metabolite, monoethyl phthalate. Arch. Toxicol. 2020, 94, 2377–2400. [Google Scholar] [CrossRef]
- Shankar, A.; Teppala, S.; Sabanayagam, C. Bisphenol A and Peripheral Arterial Disease: Results from the NHANES. Environ. Health Perspect. 2012, 120, 1297–1300. [Google Scholar] [CrossRef]
- Michałowicz, J. Bisphenol A–sources, toxicity and biotransformation. Environ. Toxicol. Pharmacol. 2014, 37, 738–758. [Google Scholar] [CrossRef]
- Santos, S.S.; Lorenzoni, A.; Ferreira, L.M.; Mattiazzi, J.; Adams, A.I.; Denardi, L.B.; Alves, S.H.; Schaffazick, S.R.; Cruz, L. Clotrimazole-loaded Eudragit® RS100 nanocapsules: Preparation, characterization and in vitro evaluation of antifungal activity against Candida species. Mater. Sci. Eng. C 2013, 33, 1389–1394. [Google Scholar] [CrossRef]
- Kolev, I.N.; Ivanova, N.; Alexieva, G.; Tsutsumanova, G.G.; Strashilov, V.L. Diltiazem-loaded Eudragit RS 100 microparticles for drug delivery: The challenge of viscosity. Scr. Sci. Pharm. 2018, 5, 20–24. [Google Scholar] [CrossRef]
- Pan, W.; Zhang, W.; Li, X.; Ye, T.; Chen, F.; Yu, S.; Chen, J.; Yang, X.; Yang, N.; Zhang, J.; et al. Nanostructured lipid carrier surface modified with Eudragit RS 100 and its potential ophthalmic functions. Int. J. Nanomed. 2014, 9, 4305–4315. [Google Scholar] [CrossRef]
- El-Nahas, A.E.; Allam, A.N.; Abdelmonsif, D.A.; El-Kamel, A.H. Silymarin-Loaded Eudragit Nanoparticles: Formulation, Characterization, and Hepatoprotective and Toxicity Evaluation. AAPS PharmSciTech 2017, 18, 3076–3086. [Google Scholar] [CrossRef]
- Almashgab, A.M.; Yahya, E.B.; Banu, A. The Cytotoxicity Effects of Outer Membrane Vesicles Isolated from Hospital and Laboratory Strains of Pseudomonas Aeruginosa on Human Keratinocyte Cell Line. Malays. J. Sci. 2020, 39, 45–53. [Google Scholar] [CrossRef]
- Menjoge, A.R.; Rinderknecht, A.L.; Navath, R.S.; Faridnia, M.; Kim, C.J.; Romero, R.; Miller, R.K.; Kannan, R.M. Transfer of PAMAM dendrimers across human placenta: Prospects of its use as drug carrier during pregnancy. J. Control. Release 2011, 150, 326–338. [Google Scholar] [CrossRef] [Green Version]
- Luong, D.; Kesharwani, P.; Deshmukh, R.; Amin, M.C.I.M.; Gupta, U.; Greish, K.; Iyer, A.K. PEGylated PAMAM dendrimers: Enhancing efficacy and mitigating toxicity for effective anticancer drug and gene delivery. Acta Biomater. 2016, 43, 14–29. [Google Scholar] [CrossRef]
- Heiden, T.C.K.; Dengler, E.; Kao, W.J.; Heideman, W.; Peterson, R.E. Developmental toxicity of low generation PAMAM dendrimers in zebrafish. Toxicol. Appl. Pharmacol. 2007, 225, 70–79. [Google Scholar] [CrossRef]
- Albertazzi, L.; Gherardini, L.; Brondi, M.; Sato, S.S.; Bifone, A.; Pizzorusso, T.; Ratto, G.M.; Bardi, G. In Vivo Distribution and Toxicity of PAMAM Dendrimers in the Central Nervous System Depend on Their Surface Chemistry. Mol. Pharm. 2012, 10, 249–260. [Google Scholar] [CrossRef]
- Wu, J.; Xie, X.; Zheng, Z.; Li, G.; Wang, X.; Wang, Y. Effect of pH on polyethylene glycol (PEG)-induced silk microsphere formation for drug delivery. Mater. Sci. Eng. C 2017, 80, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Smyth, H.F.J.; Carpenter, C.P.; Weil, C.S. The toxicology of the polyethylene glycols. J. Am. Pharm. Assoc. (Sci. Ed.) 1950, 39, 349–354. [Google Scholar] [CrossRef]
- Liu, G.; Li, Y.; Yang, L.; Wei, Y.; Wang, X.; Wang, Z.; Tao, L. Cytotoxicity study of polyethylene glycol derivatives. RSC Adv. 2017, 7, 18252–18259. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Zou, Y.; Wu, P.; Meng, J.; Zhang, R. Phthalate exposure in pregnant women and the influence of exposure to environmental tobacco smoke. J. Matern. Neonatal Med. 2019, 33, 3111–3115. [Google Scholar] [CrossRef]
- Broe, A.; Pottegård, A.; Hallas, J.; Ahern, T.P.; Lamont, R.F.; Damkier, P. Phthalate exposure from drugs during pregnancy and possible risk of preterm birth and small for gestational age. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 240, 293–299. [Google Scholar] [CrossRef]
- Ferguson, K.K.; McElrath, T.F.; Meeker, J.D. Environmental Phthalate Exposure and Preterm Birth. JAMA Pediatr. 2014, 168, 61–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marie, C.; Vendittelli, F.; Sauvant-Rochat, M.-P. Obstetrical outcomes and biomarkers to assess exposure to phthalates: A review. Environ. Int. 2015, 83, 116–136. [Google Scholar] [CrossRef] [PubMed]
- Smeak, D.D. Suture Materials, Staplers, and Tissue Apposition Devices. In Gastrointestinal Surgical Techniques in Small Animals; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2020; pp. 9–21. [Google Scholar] [CrossRef]
- Yahya, E.B.; Alzalouk, M.M.; Alfallous, K.A.; Abogmaza, A.F. Antibacterial cellulose-based aerogels for wound healing application: A review. Biomed. Res. Ther. 2020, 7, 4032–4040. [Google Scholar] [CrossRef]
- Lee, J.; Oh, S.; Jeon, M.J. Suture Complication Rates and Surgical Outcomes According to the Nonabsorbable Suture Materials Used in Vaginal Uterosacral Ligament Suspension: Polyester vs Polypropylene. J. Minim. Invasive Gynecol. 2020, in press. [Google Scholar] [CrossRef]
- Kim, H.; Kim, B.H.; Huh, B.K.; Yoo, Y.C.; Heo, C.Y.; Bin Choy, Y.; Park, J.-H. Surgical suture releasing macrophage-targeted drug-loaded nanoparticles for an enhanced anti-inflammatory effect. Biomater. Sci. 2017, 5, 1670–1677. [Google Scholar] [CrossRef]
- Lee, D.-H.; Kwon, T.-Y.; Kim, K.-H.; Kwon, S.-T.; Cho, D.-H.; Jang, S.H.; Son, J.S.; Lee, K.-B. Anti-inflammatory drug releasing absorbable surgical sutures using poly(lactic-co-glycolic acid) particle carriers. Polym. Bull. 2014, 71, 1933–1946. [Google Scholar] [CrossRef]
- Ceonzo, K.; Gaynor, A.; Shaffer, L.; Kojima, K.; Vacanti, C.A.; Stahl, G.L. Polyglycolic acid-induced inflammation: Role of hydrolysis and resulting complement activation. Tissue Eng. 2006, 12, 301–308. [Google Scholar] [CrossRef]
- Junge, K. Gentamicin supplementation of polyvinylidenfluoride mesh materials for infection prophylaxis. Biomaterials 2005, 26, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Al-Mubarak, L.; Al-Haddab, M. Cutaneous wound closure materials: An overview and update. J. Cutan. Aesthetic Surg. 2013, 6, 178–188. [Google Scholar] [CrossRef]
- Tan, R.H.H.; Bell, R.J.W.; Dowling, B.A.; Dart, A.J. Suture materials: Composition and applications in veternary wound repair. Aust. Vet. J. 2003, 81, 140–145. [Google Scholar] [CrossRef]
- Curet, M.J. Special problems in laparoscopic surgery: Previous abdominal surgery, obesity, and pregnancy. Surg. Clin. N. Am. 2000, 80, 1093–1110. [Google Scholar] [CrossRef]
- Shi, Q.; Xie, Y.; Wang, Y.; Li, S. Vitrification versus slow freezing for human ovarian tissue cryopreservation: A systematic review and meta-anlaysis. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef]
- Larman, M.G.; Minasi, M.G.; Rienzi, L.; Gardner, D.K. Maintenance of the meiotic spindle during vitrification in human and mouse oocytes. Reprod. Biomed. Online 2007, 15, 692–700. [Google Scholar] [CrossRef]
- Faustino, L.R.; de Andrade Carvalho, A.; Da Silva, C.M.G.; de Figueiredo, J.R.; Rodrigues, A.P.R. Criopreservação de tecido ovariano: Limitações e perspectivas para a preservação da fertilidade de fêmeas. Acta Sci. Vet. 2011, 39, 1–15. [Google Scholar]
- Bari, J.; Islam, M.N.; Alam, M.H.; Khatun, A.; Hashem, M.A.; Moniruzzaman, M. Effect of Polyvinylpyrrolidone on Vitrification of Buffalo (Bubalus bubalis) Oocytes. J. Buffalo Sci. 2020, 9, 152–158. [Google Scholar] [CrossRef]
- Amorim, C.A.; David, A.; Van Langendonckt, A.; Dolmans, M.-M.; Donnez, J. Vitrification of human ovarian tissue: Effect of different solutions and procedures. Fertil. Steril. 2011, 95, 1094–1097. [Google Scholar] [CrossRef]
- Kokotsaki, M.; Mairhofer, M.; Schneeberger, C.; Marschalek, J.; Pietrowski, D. Impact of vitrification on granulosa cell survival and gene expression. Cryobiology 2018, 85, 73–78. [Google Scholar] [CrossRef]
- Santos, N.C.; Figueira-Coelho, J.; Martins-Silva, J.; Saldanha, C. Multidisciplinary utilization of dimethyl sulfoxide: Pharmacological, cellular, and molecular aspects. Biochem. Pharmacol. 2003, 65, 1035–1041. [Google Scholar] [CrossRef]
- Berge, T.L.L. Treatment with Dental Polymer-Based Restorative Materials: Exposure to Bisphenol A Effects on Pregnancy Outcomes; The University of Bergen: Bergen, Norway, 2019. [Google Scholar]
- Berge, T.L.L.; Lygre, G.B.; Lie, S.A.; Björkman, L. Polymer-based dental filling materials placed during pregnancy and risk to the foetus. BMC Oral Health 2018, 18, 144. [Google Scholar] [CrossRef] [PubMed]
- Richter, C.A.; Birnbaum, L.S.; Farabollini, F.; Newbold, R.R.; Rubin, B.S.; Talsness, C.E.; Vandenbergh, J.G.; Walser-Kuntz, D.R.; Saal, F.S.V. In vivo effects of bisphenol A in laboratory rodent studies. Reprod. Toxicol. 2007, 24, 199–224. [Google Scholar] [CrossRef] [Green Version]
- Vandenberg, L.N.; Colborn, T.; Hayes, T.B.; Heindel, J.J.; Jacobs, D.R.; Lee, D.-H.; Shioda, T.; Soto, A.M.; Saal, F.S.V.; Welshons, W.V.; et al. Hormones and Endocrine-Disrupting Chemicals: Low-Dose Effects and Nonmonotonic Dose Responses. Endocr. Rev. 2012, 33, 378–455. [Google Scholar] [CrossRef]
- Pfeifer, C.S. Polymer-Based Direct Filling Materials. Dent. Clin. N. Am. 2017, 61, 733–750. [Google Scholar] [CrossRef] [PubMed]
- Nuryawan, A.; Abdullah, C.K.; Hazwan, C.M.; Olaiya, N.G.; Yahya, E.B.; Risnasari, I.; Masruchin, N.; Baharudin, M.S.; Khalid, H.; Khalil, H.P.S.A. Enhancement of Oil Palm Waste Nanoparticles on the Properties and Characterization of Hybrid Plywood Biocomposites. Polymers 2020, 12, 1007. [Google Scholar] [CrossRef]
- Khalil, H.P.S.A.; Jummaat, F.; Yahya, E.B.; Olaiya, N.G.; Adnan, A.S.; Abdat, M.; Nasir, N.A.M.; Halim, A.S.; Kumar, U.S.U.; Bairwan, R.; et al. A Review on Micro- to Nanocellulose Biopolymer Scaffold Forming for Tissue Engineering Applications. Polymers 2020, 12, 2043. [Google Scholar] [CrossRef] [PubMed]
- Abogmaza, A.F.; Keer, K.F.; Takrizzah, A.A.; Yahya, E.B. A Review on the Medicinal and Aromatic Plants Growing in Libya and Their Therapeutic Properties. Int. Res. J. Sci. Technol. 2020, 327–334. [Google Scholar] [CrossRef]
- Balasundaram, G.; Webster, T.J. An Overview of Nano-Polymers for Orthopedic Applications. Macromol. Biosci. 2007, 7, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Ramphul, H.; Gimié, F.; Andries, J.; Jhurry, D.; Bhaw-Luximon, A. Sugar-cane bagasse cellulose-based scaffolds promote multi-cellular interactions, angiogenesis and reduce inflammation for skin tissue regeneration. Int. J. Biol. Macromol. 2020, 157, 296–310. [Google Scholar] [CrossRef]
- Vartiainen, J.; Pöhler, T.; Sirola, K.; Pylkkänen, L.; Alenius, H.; Hokkinen, J.; Tapper, U.; Kapanen, A.; Lahtinen, P.; Laukkanen, A.; et al. Health and environmental safety aspects of friction grinding and spray drying of microfibrillated cellulose. Cellulose 2011, 18, 775–786. [Google Scholar] [CrossRef]
- Teo, A.J.; Mishra, A.; Park, I.; Kim, Y.-J.; Park, W.-T.; Yoon, Y.-J. Polymeric Biomaterials for Medical Implants and Devices. ACS Biomater. Sci. Eng. 2016, 2, 454–472. [Google Scholar] [CrossRef]
- Peterson, D.R. Biomaterials: Principles and Practices; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Hiorth, M.; Nilsen, S.; Tho, I. Bioadhesive Mini-Tablets for Vaginal Drug Delivery. Pharmaceutics 2014, 6, 494–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rebelo, R.; Fernandes, M.; Fangueiro, R. Biopolymers in Medical Implants: A Brief Review. Procedia Eng. 2017, 200, 236–243. [Google Scholar] [CrossRef]
- Pattanashetti, N.A.; Heggannavar, G.B.; Kariduraganavar, M.Y. Smart Biopolymers and their Biomedical Applications. Procedia Manuf. 2017, 12, 263–279. [Google Scholar] [CrossRef]
- Yahya, E.; AbdulSamad, M.A. In-vitro Antibacterial Activity of Carbopol-Essential Oils hydrogels. J. Appl. Sci. Process. Eng. 2020, 7, 564–571. [Google Scholar] [CrossRef]
- Sionkowska, A.; Kaczmarek, B.; Michalska, M.; Lewandowska, K.; Grabska, S. Preparation and characterization of collagen/chitosan/hyaluronic acid thin films for application in hair care cosmetics. Pure Appl. Chem. 2017, 89, 1829–1839. [Google Scholar] [CrossRef]
- Schueller, R.; Romanowski, P. Multifunctional Cosmetics; Marcel Dekker Inc.: New York, NY, USA, 2001. [Google Scholar]
- Jiang, Y.; Liu, L.; Wang, B.; Yang, X.; Chen, Z.; Zhong, Y.; Zhang, L.; Mao, Z.; Xu, H.; Sui, X. Polysaccharide-based edible emulsion gel stabilized by regenerated cellulose. Food Hydrocoll. 2019, 91, 232–237. [Google Scholar] [CrossRef]
- Krstonošić, V.; Dokić, L.; Nikolić, I.; Milanović, M. Influence of xanthan gum on oil-in-water emulsion characteristics stabilized by OSA starch. Food Hydrocoll. 2015, 45, 9–17. [Google Scholar] [CrossRef]
- Shakeri-Zadeh, A.; Bashari, A.; Kamrava, S.K.; Ghalehbaghi, S. The Use of Hydrogel/Silver Nanoparticle System for Preparation of New Type of Feminine Tampons. BioNanoScience 2016, 6, 284–292. [Google Scholar] [CrossRef]
- Rowley, J.; Hoorn, S.V.; Korenromp, E.; Low, N.; Unemo, M.; Abu-Raddad, L.J.; Chico, R.M.; Smolak, A.; Newman, L.; Gottlieb, S.; et al. Chlamydia, gonorrhoea, trichomoniasis and syphilis: Global prevalence and incidence estimates, 2016. Bull. World Health Organ. 2019, 97, 548–562. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Quito, E.-M.; Ruiz-Caro, R.; Rubio, J.; Tamayo, A.; Veiga, M.-D. Carrageenan-Based Acyclovir Mucoadhesive Vaginal Tablets for Prevention of Genital Herpes. Mar. Drugs 2020, 18, 249. [Google Scholar] [CrossRef] [PubMed]
- Kozlowska, J.; Stachowiak, N.; Sionkowska, A. Collagen/Gelatin/Hydroxyethyl Cellulose Composites Containing Microspheres Based on Collagen and Gelatin: Design and Evaluation. Polymers 2018, 10, 456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brigham, C. Chitin and Chitosan: Sustainable, Medically Relevant Biomaterials. Int. J. Biotechnol. Wellness Ind. 2017, 6, 41–47. [Google Scholar] [CrossRef]
- Yan, C.; Pochan, D.J. Rheological properties of peptide-based hydrogels for biomedical and other applications. Chem. Soc. Rev. 2010, 39, 3528–3540. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Z.; Ling, S.; Yeo, J.; Zhao, S.; Tozzi, L.; Buehler, M.J.; Omenetto, F.; Li, C.; Kaplan, D.L. High-Strength, Durable All-Silk Fibroin Hydrogels with Versatile Processability toward Multifunctional Applications. Adv. Funct. Mater. 2018, 28. [Google Scholar] [CrossRef]
- Ullah, H.; Santos, H.A.; Khan, T. Applications of bacterial cellulose in food, cosmetics and drug delivery. Cellulose 2016, 23, 2291–2314. [Google Scholar] [CrossRef]
- Aranaz, I.; Acosta, N.; Civera, C.; Elorza, B.; Mingo, J.; Castro, C.; Gandía, M.D.L.L.; Caballero, A.H. Cosmetics and Cosmeceutical Applications of Chitin, Chitosan and Their Derivatives. Polymers 2018, 10, 213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graziola, F.; Candido, T.M.; Oliveira, C.A.D.; Peres, D.D.A.; Issa, M.G.; Mota, J.; Rosado, C.; Consiglieri, V.O.; Kaneko, T.M.; Baby, A.R.; et al. Gelatin-based microspheres cross-linked with glutaraldehyde and rutin oriented to cosmetics. Braz. J. Pharm. Sci. 2016, 52, 603–612. [Google Scholar] [CrossRef] [Green Version]
- Bukhari, S.N.A.; Roswandi, N.L.; Waqas, M.; Habib, H.; Hussain, F.; Khan, S.; Sohail, M.; Ramli, N.A.; Thu, H.E.; Hussain, Z. Hyaluronic acid, a promising skin rejuvenating biomedicine: A review of recent updates and pre-clinical and clinical investigations on cosmetic and nutricosmetic effects. Int. J. Biol. Macromol. 2018, 120, 1682–1695. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.L.; Marques, A.L.P.; Martins, E.; Silva, T.H.; Reis, R.L. Cosmetic Potential of Marine Fish Skin Collagen. Cosmetics 2017, 4, 39. [Google Scholar] [CrossRef] [Green Version]
- Song, J.; Chen, H. Preparation of aroma microcapsules with sodium alginate and tetradecylallyldimethylammonium bromide (TADAB) and its potential applications in cosmetics. Flavour Fragr. J. 2018, 33, 160–165. [Google Scholar] [CrossRef]
- Can, U.; Tuncer, M.; Narter, F.; Sabuncu, K.; Sarıca, K. Ureteral Stent Use in Pregnant Women with Persistent Flank Pain: Our Clinical Experience. South. Clin. Istanb. Eurasia 2018, 29, 285–289. [Google Scholar]
- Škrlová, K.; Malachová, K.; Muñoz-Bonilla, A.; Měřinská, D.; Rybková, Z.; Fernández-García, M.; Plachá, D. Biocompatible polymer materials with antimicrobial properties for preparation of stents. Nanomaterials 2019, 9, 1548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cazorla-Luna, R.; Notario-Pérez, F.; Martín-Illana, A.; Bedoya, L.-M.; Tamayo, A.; Rubio, J.; Ruiz-Caro, R.; Veiga, M.-D. Development and In Vitro/Ex Vivo Characterization of Vaginal Mucoadhesive Bilayer Films Based on Ethylcellulose and Biopolymers for Vaginal Sustained Release of Tenofovir. Biomacromolecules 2020, 21, 2309–2319. [Google Scholar] [CrossRef]
- Tuğcu-Demiröz, F. Development of in situ poloxamer-chitosan hydrogels for vaginal drug delivery of benzydamine hydrochloride: Textural, mucoadhesive and in vitro release properties. Marmara Pharm. J. 2017, 21, 762–770. [Google Scholar] [CrossRef] [Green Version]
- Parvinroo, S.; Eslami, M.; Ebrahimi-Najafabadi, H.; Hesari, Z. Natural polymers for vaginal mucoadhesive delivery of vinegar, using design of experiment methods. Vojn. Pregl. 2020, 121. [Google Scholar] [CrossRef]
- Gómez-Mascaraque, L.G.; Fabra, M.J.; Castro-Mayorga, J.L.; Sánchez, G.; Martínez-Sanz, M.; López-Rubio, A. Nanostructuring Biopolymers for Improved Food Quality and Safety. In Biopolymers for Food Design; Academic Press: Cambridge, MA, USA, 2018; pp. 33–64. [Google Scholar] [CrossRef]
- Jacob, J.; Haponiuk, J.T.; Thomas, S.; Gopi, S. Biopolymer based nanomaterials in drug delivery systems: A review. Mater. Today Chem. 2018, 9, 43–55. [Google Scholar] [CrossRef]
- Cheng, L.; Norenhag, J.; Hu, Y.O.O.; Brusselaers, N.; Fransson, E.; Ährlund-Richter, A.; Guðnadóttir, U.; Angelidou, P.; Zha, Y.; Hamsten, M.; et al. Vaginal microbiota and human papillomavirus infection among young Swedish women. Npj Biofilms Microbiomes 2020, 6, 1–10. [Google Scholar] [CrossRef]
- Enin, A.S.A.E.; Elbakry, A.M.; El Hosary, R.; Lotfy, M.A.F.; Yahia, R. Formulation, development, in vivo pharmacokinetics and pharmacological efficacy evaluation of novel vaginal bioadhesive sustained core-in-cup salbutamol sulphate tablets for preterm labor. J. Drug Deliv. Sci. Technol. 2020, 60, 102076. [Google Scholar] [CrossRef]
- Pandey, M.; Choudhury, H.; Abdul-Aziz, A.; Bhattamisra, S.K.; Gorain, B.; Carine, T.; Toong, T.W.; Yi, N.J.; Yi, L.W. Promising Drug Delivery Approaches to Treat Microbial Infections in the Vagina: A Recent Update. Polymers 2020, 13, 26. [Google Scholar] [CrossRef]
- Kuna, M.; Waller, J.P.; Logue, O.C.; Bidwell, G.L. Polymer size affects biodistribution and placental accumulation of the drug delivery biopolymer elastin-like polypeptide in a rodent pregnancy model. Placenta 2018, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, J.A. The Use of Barbed Sutures in Obstetrics and Gynecology. Rev. Obstet. Gynecol. 2010, 3, 82–91. [Google Scholar]
- Levin, S.R.; De Geus, S.W.; Noel, N.L.; Paasche-Orlow, M.K.; Farber, A.; Siracuse, J.J. Vascular repairs in gynecologic operations are uncommon but predict major morbidity and mortality. J. Vasc. Surg. 2020, 72, 1059–1066.e2. [Google Scholar] [CrossRef]
- Joseph, B.; George, A.; Gopi, S.; Kalarikkal, N.; Thomas, S. Polymer sutures for simultaneous wound healing and drug delivery—A review. Int. J. Pharm. 2017, 524, 454–466. [Google Scholar] [CrossRef]
- Serrano, C.; García-Fernández, L.; Fernández-Blázquez, J.P.; Barbeck, M.; Ghanaati, S.; Unger, R.; Kirkpatrick, J.; Arzt, E.; Funk, L.; Turón, P.; et al. Nanostructured medical sutures with antibacterial properties. Biomaterials 2015, 52, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Park, S.H.; Lee, J.H.; Jeong, B.Y.; Ahn, S.K.; Choi, Y.M.; Choi, D.J.; Chang, J.H. Antimicrobial and biodegradable PLGA medical sutures with natural grapefruit seed extracts. Mater. Lett. 2013, 95, 40–43. [Google Scholar] [CrossRef]
- Chandrasekaran, N.; Dhas, S.P.; Anbarasan, S.; Mukherjee, A. Biobased silver nanocolloid coating on silk fibers for prevention of post-surgical wound infections. Int. J. Nanomed. 2015, 10, 159–170. [Google Scholar] [CrossRef] [Green Version]
- Kawamura, A.; Yoshioka, D.; Toda, K.; Sakaniwa, R.; Miyagawa, S.; Yoshikawa, Y.; Hata, H.; Shimamura, K.; Kin, K.; Kainuma, S.; et al. An evaluation of the long-term patency of the aortocoronary bypass graft anastomosed to a vascular prosthesis. Eur. J. Cardio-Thorac. Surg. 2020. [Google Scholar] [CrossRef] [PubMed]
- Stanirowski, P.; Sawicki, W. Modern methods of therapy of hard-to-heal post-operative wounds in obstetrics and gynecology–Analysis of applicability and effectiveness of use. Postępy Nauk Med. 2013, 7, 475–487. [Google Scholar]
- Mangram, A.J.; Horan, T.C.; Pearson, M.L.; Silver, L.C.; Jarvis, W.R. The Hospital Infection Control Practices Advisory Committee Guideline for Prevention of Surgical Site Infection, 1999. Infect. Control. Hosp. Epidemiol. 1999, 20, 247–280. [Google Scholar] [CrossRef]
- Plowman, R.; Graves, N.; Griffin, M.; Roberts, J.; Swan, A.; Cookson, B.; Taylor, L. The rate and cost of hospital-acquired infections occurring in patients admitted to selected specialties of a district general hospital in England and the national burden imposed. J. Hosp. Infect. 2001, 47, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.; Thakar, R.; Sultan, A.H. Obstetric perineal wound infection: Is there underreporting? Br. J. Nurs. 2012, 21. [Google Scholar] [CrossRef]
- Wloch, C.; Wilson, J.; Lamagni, T.; Harrington, P.; Charlett, A.; Sheridan, E. Risk factors for surgical site infection following caesarean section in England: Results from a multicentre cohort study. BJOG Int. J. Obstet. Gynaecol. 2012, 119, 1324–1333. [Google Scholar] [CrossRef]
- Yerushalmy, A.; Reches, A.; Lessing, J.B.; Schechner, V.; Carmeli, Y.; Grisaru, D. Characteristics of microorganisms cultured from infected wounds post-hysterectomy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008, 141, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Wills, A.; Obermair, A. A review of complications associated with the surgical treatment of vulvar cancer. Gynecol. Oncol. 2013, 131, 467–479. [Google Scholar] [CrossRef]
- Nilsson, L.; Wodlin, N.B.; Kjølhede, P. Risk factors for postoperative complications after fast-track abdominal hysterectomy. Aust. N. Z. J. Obstet. Gynaecol. 2012, 52, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Stanirowski, P.; Wnuk, A.; Cendrowski, K.; Sawicki, W. Growth factors, silver dressings and negative pressure wound therapy in the management of hard-to-heal postoperative wounds in obstetrics and gynecology: A review. Arch. Gynecol. Obstet. 2015, 292, 757–775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Dillen, J.; Zwart, J.; Schutte, J.; van Roosmalen, J. Maternal sepsis: Epidemiology, etiology and outcome. Curr. Opin. Infect. Dis. 2010, 23, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Fouda, M.M.; Wittke, R.; Knittel, D.; Schollmeyer, E. Use of chitosan/polyamine biopolymers based cotton as a model system to prepare antimicrobial wound dressing. Int. J. Diabetes Mellit. 2009, 1, 61–64. [Google Scholar] [CrossRef] [Green Version]
- Kamoun, E.A.; Kenawy, E.-R.S.; Chen, X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J. Adv. Res. 2017, 8, 217–233. [Google Scholar] [CrossRef]
- McCandlish, R.; Bowler, U.; Asten, H.; Berridge, G.; Winter, C.; Sames, L.; Garcia, J.; Renfrew, M.; Elbourne, D. A randomised controlled trial of care of the perineum during second stage of normal labour. BJOG: Int. J. Obstet. Gynaecol. 1998, 105, 1262–1272. [Google Scholar] [CrossRef]
- Gokarneshan, N. Effectiveness of Textile Materials in Gynaecology and Obstetrics. Open Acc. J. Reprod. Sex. Disord. 2018, 1. [Google Scholar] [CrossRef]
- Childs, C.; Sandy-Hodgetts, K.; Broad, C.; Cooper, R.; Manresa, M.; Verdú-Soriano, J. Risk, Prevention and Management of Complications After Vaginal and Caesarean Section Birth. J. Wound Care 2020, 29, S1–S48. [Google Scholar] [CrossRef]
- Surya, I.; Olaiya, N.G.; Rizal, S.; Zein, I.; Aprilia, N.A.S.; Hasan, M.; Yahya, E.B.; Sadasivuni, K.K.; Khalil, H.P.S.A. Plasticizer Enhancement on the Miscibility and Thermomechanical Properties of Polylactic Acid-Chitin-Starch Composites. Polymers 2020, 12, 115. [Google Scholar] [CrossRef] [Green Version]
- Ostovan, A.; Ghaedi, M.; Arabi, M.; Yang, Q.; Li, J.; Chen, L. Hydrophilic Multitemplate Molecularly Imprinted Biopolymers Based on a Green Synthesis Strategy for Determination of B-Family Vitamins. ACS Appl. Mater. Interfaces 2018, 10, 4140–4150. [Google Scholar] [CrossRef]








| Biopolymer | Advantages | Disadvantages | Ref |
|---|---|---|---|
| Cellulose | Improve the moisture in the skin and minimizes hyper-pigmentation appearance. | Poor compatibility with hydrophobic matrixes. | [120] |
| Chitosan | Strong antimicrobial, antioxidant properties, as well as softens the skin. | Chitosan intrinsic properties may be affected by its cross-linking. | [121] |
| Gelatin | Improving skin health and significantly cause skin firmness. | Potential allergic reactions in some individuals. | [122] |
| Hyaluronic acid | Reduction of wrinkles and visibility of fine lines, as well as smoothening the skin. | Rash on the application site and potential allergic reactions. | [123] |
| Collagen | Reduces skin wrinkles, improves its elasticity, and boosts skin hydration. | Possible inflammation responses in some individuals. | [124] |
| Alginate | Improve skin elasticity, strengthens and freshens, as well as erasing fine wrinkles. | Some formulations may have a foul smell. | [125] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jummaat, F.; Yahya, E.B.; Khalil H.P.S., A.; Adnan, A.S.; Alqadhi, A.M.; Abdullah, C.K.; A.K., A.S.; Olaiya, N.G.; Abdat, M. The Role of Biopolymer-Based Materials in Obstetrics and Gynecology Applications: A Review. Polymers 2021, 13, 633. https://doi.org/10.3390/polym13040633
Jummaat F, Yahya EB, Khalil H.P.S. A, Adnan AS, Alqadhi AM, Abdullah CK, A.K. AS, Olaiya NG, Abdat M. The Role of Biopolymer-Based Materials in Obstetrics and Gynecology Applications: A Review. Polymers. 2021; 13(4):633. https://doi.org/10.3390/polym13040633
Chicago/Turabian StyleJummaat, Fauziah, Esam Bashir Yahya, Abdul Khalil H.P.S., A. S. Adnan, Amaal Mohammed Alqadhi, C. K. Abdullah, Atty Sofea A.K., N. G. Olaiya, and Munifah Abdat. 2021. "The Role of Biopolymer-Based Materials in Obstetrics and Gynecology Applications: A Review" Polymers 13, no. 4: 633. https://doi.org/10.3390/polym13040633