Biopolymer Composites as an Alternative to Materials for the Production of Ecological Packaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Research Facilities
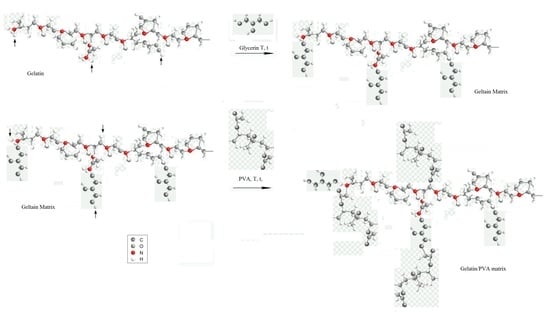
2.2. Preparation of the Composites
2.3. Selection of Matrix Composition
2.4. Preparation of Compositions Based on Gelatin
2.5. Introduction of the Filler
2.6. Research Techniques
3. Results and Discussion
3.1. Characterization of Base Composites
3.1.1. Shore Hardness Testing
3.1.2. Breaking Strength
3.1.3. FTIR Spectroscopic Analysis
3.1.4. Microscopic Research (SEM)
3.2. Characterization of the Composites with Fillers
3.2.1. Shore Hardness Testing and Breaking Strength
3.2.2. FTIR Analysis
3.2.3. DSC and TGA Analysis
3.2.4. Analysis of the Contact Angle and SFE (Surface Free Energy)
3.2.5. Measurement of the Equilibrium Swelling
3.2.6. SEM and EDS Analysis of Composites
3.2.7. Determination of Resistance to Thermo-Oxidative Aging, Biodegradation, and Color Change of Composites
Thermo-Oxidative Aging
Determining the Susceptibility of the Composites to Biodegradation
Determination of Color Changes after Aging Processes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ray, D. Biocomposites for High.-Performance Applications: Current Barriers and Future Needs Towards Industrial Development; Woodhead Publishing: Cambridge, UK, 2017; ISBN: 9780081007938 or 9780081007945. [Google Scholar]
- Lawrence, P. Bio-based and biodegradable plastics, An annotated selection of World Wide Web sites relevant to the topics in microbial biotechnology. Microb. Biotechnol. 2019, 12, 1492–1493. [Google Scholar]
- Ashter, S.A. Introduction to Bioplastics Engineering; William Andrew: Norwich, NY, USA, 2016; ISBN: 9780323393966 or 9780323394079. [Google Scholar]
- Grumezescu, A. Food Packaging; Academic Press: Cambridge, MA, USA, 2016; ISBN: 9780128043028 or 9780128043738. [Google Scholar]
- Farmer, N. Trends in packaging of food, beverages and other fast-moving consumer goods (FMCG). In Trends in Packaging of Food, Beverages and Other Fast-Moving Consumer Goods (FMCG); Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Maragkaki, A.; Sampathianakis, I.; Katrini, K.; Michalodimitraki, E.; Gryparis, C.; Raptis, V.; Power, A.; Lolos, T.; Tsobanidis, C.; Harmandaris, V.; et al. Bio-waste to Bio-plastic (B2B): Production of Compostable Bio-Plastics from Food Waste. Multidiscip. Digit. Publ. Inst. Proc. 2019, 30, 47. [Google Scholar] [CrossRef] [Green Version]
- Tsou, C.-Y.; Suen, M.-C.; Yao, W.-H.; Yeh, J.-T.; Wu, C.-S.; Chiu, S.-H.; Chen, J.-C.; Wang, R.Y.; Lin, S.-M.; Hung, W.-S.; et al. Preparation and Characterization of Bioplastic-Based Green Renewable Composites from Tapioca with Acetyl Tributyl Citrate as a Plasticizer. Materials 2014, 7, 5617–5632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marichelvam, M.K.; Jawaid, M.; Asim, M. Corn and Rice Starch-Based Bio-Plastics as Alternative Packaging Materials. Fibers 2019, 7, 32. [Google Scholar] [CrossRef] [Green Version]
- Giosafatto, C.V.L.; Al-Asmar, A.; D’Angelo, A.; Roviello, V.; Esposito, M.; Mariniello, L. Preparation and Characterization of Bioplastics from Grass Pea Flour Cast in the Presence of Microbial Transglutaminase. Coatings 2018, 8, 435. [Google Scholar] [CrossRef] [Green Version]
- Neves, A.C.; Moyne, M.M.; Eyre, C.; Casey, B.P. Acceptability and Societal Impact of the Introduction of Bioplastics as Novel Environmentally Friendly Packaging Materials in Ireland. Clean Technol. 2020, 2, 9. [Google Scholar] [CrossRef] [Green Version]
- Wright, S.L.; Kelly, F.J. Plastic and Human Health: A Micro Issue? Environ. Sci. Technol. 2017, 51, 6634–6647. [Google Scholar] [CrossRef]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Przygocki, W.; Włochowicz, A. Fizyka Polimerów; Państwowe Wydawnictwo Naukowe PWN: Warszawa, Poland, 2001. [Google Scholar]
- John, L.; Kice, E.; Marvell, N. Zarys Chemii Organicznej; Państwowe Wydawnictwo Naukowe PWN: Warszawa, Poland, 2001. [Google Scholar]
- Lide, D.R.; Milne, G.W. Handbook of Data on Organic Compounds; CRC Press, Inc.: BocaRaton, FL, USA, 1994; Volume 1, p. 4386. [Google Scholar]
- Grondahl, M.; Gustafsson, A.; Gatenholm, P. Gas-phase surface flourination of arabinoxlyan films. Macromolecules 2006, 39, 2718–2721. [Google Scholar] [CrossRef]
- Wackett, L.P. Polylactic acid (PLA). Microb. Biotechnol. 2008, 1, 432–433. [Google Scholar] [CrossRef]
- Dorgan, J.R.; Lehermeier, H.; Mang, M. Thermal and Rheological Properties of Commercial-Grade Poly(Lactic Acid)s. J. Polym. Environ. 2000, 8, 1–9. [Google Scholar] [CrossRef]
- Chiellini, E. Enviromentally compatible food packaging; Woodhead Publishing: Cambridge, UK, 2008; ISBN 978-1-84569-19. [Google Scholar]
- Kurcok, P.; Kawalec, M.; Sobota, M.; Michalak, M.; Kwiecien, M.; Jurczyk, S. Polyhydroxyalkanoates—applications and recycling. Polimery 2017, 62, 364–370. [Google Scholar] [CrossRef]
- Berezina, N.; Martelli, S.M. CHAPTER 1. Bio-based Polymers and Materials. Microw. Assist. Polym. 2014, 1, 1–28. [Google Scholar] [CrossRef]
- Müller, R. Biodegradability of Polymers: Regulations and Methods for Testing; Wiley: Hoboken, NJ, USA, 2002; p. 365. [Google Scholar]
- Haider, T.P.; Völker, C.; Kramm, J.; Landfester, K.; Wurm, F.R. Plastics of the Future? The Impact of Biodegradable Polymers on the Environment and on Society. Angew. Chem. Int. Ed. 2019, 58, 50–62. [Google Scholar] [CrossRef] [Green Version]
- Adamcová, D.; Fronczyk, J.; Radziemska, M.; Vaverková, M.D.; Zloch, J. Research of the biodegradability of degradable/biodegradable plastic material in various types of environments. Przegląd Nauk. Inżynieria I Kształtowanie Środowiska 2017, 26, 3–14. [Google Scholar] [CrossRef]
- Babinsky, R. PVC—Additives—A Global Review. J. Vinyl Addit. Technol. 2007, 13, 1. [Google Scholar] [CrossRef]
- Żuchowska, D. Polimery Konstrukcyjne; WNT: Warszawa, Poland, 2000. [Google Scholar]
- Liao, C.-J.; Chen, C.-F.; Chen, J.-H.; Chiang, S.-F.; Lin, Y.-J.; Chang, K.-Y. Fabrication of porous biodegradable polymer scaffolds using a solvent merging/particulate leaching method. J. Biomed. Mater. Res. 2002, 59, 676–681. [Google Scholar] [CrossRef]
- Hossan, M.J.; Gafur, M.A.; Karim, M.M.; Rana, A.A. Mechanical properties of gelatin–hydroxyapatite composite for bone tissue engineering. Bangladesh J. Sci. Ind. Res. 2015, 50, 15–20. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Kennedy, J.F.; Jiang, Q.G.; Xie, B.J. Quick dissolvable, edible and heatstable blend films based on konjac glukoman-nan-gelatin. Food Res. Int. 2006, 39, 544–549. [Google Scholar] [CrossRef]
- Prochoń, M.; Marzec, A.; Szadkowski, B. Preparation and Characterization of New Environmentally Friendly Starch-Cellulose Materials Modified with Casein or Gelatin for Agricultural Applications. Materials 2019, 12, 1684. [Google Scholar] [CrossRef] [Green Version]
- Van Soest, J.G.; de Wit, D.; Tournois, H.I.; Vliegenthort, F.G. Retrogradation of potato starch as studied by Fourier transform infrared spectroscopy. Starch/Stärke 1994, 46, 453–457. [Google Scholar] [CrossRef] [Green Version]
- Pozo, C.; Rodríguez-Llamazares, S.; Bouza, R.; Barral, L.; Castaño, J.; Müller, N.; Restrepo, I. Study of the structural order of native starch granules using combined FTIR and XRD analysis. J. Poly. Res. 2018, 25, 266. [Google Scholar] [CrossRef]
- Szyk-Warszyńska, L.; Raszka, K.; Warszyński, P. Interactions of Casein and Polypeptides in Multilayer Films Studied by FTIR and Molecular Dynamics. Polymer 2019, 11, 920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukherjee, I.; Rosolen, M. Thermal transitions of gelatin evaluated using DSC sample pans of various seal integrities. J. Therm. Anal. Calorim. 2013, 114, 1161–1166. [Google Scholar] [CrossRef]
- Żenkiewicz, M. Analiza głównych metod badania swobodnej energii powierzchniowej materiałów polimerowych. Nat. Mater. 2007, 6, 760–767. [Google Scholar]
- Liber-Kneć, A.; Łagan, S. Zastosowanie pomiarów kąta zwilżania i swobodnej energii powierzchniowej do charakterystyki powierzchni polimerów wykorzystywanych w medycynie. Polim. Med. 2014, 44, 29–37. [Google Scholar] [PubMed]














| Composition | Ingredients/PBW | ||||
|---|---|---|---|---|---|
| Gelatine [PBW] | Glycerine [PBW] | PVA [PBW] | Starch [PBW] | Distilled Water [PBW] | |
| G.G.PVA.20 | 20 | 10 | 50 | - | 20 |
| G.PVA.S | - | 20 | 8 | 20 | 60 |
| G.G.75 | 75 | 25 | - | - | 83 |
| G.G.70 | 70 | 30 | - | - | 78 |
| G.G.65 | 65 | 45 | - | - | 72 |
| G.G.50 | 50 | 10 | 0 | 0 | 125 |
| Composition | Gelatine G [PBW] | Glycerine G [PBW] | Starch S [PBW] | Casein C [PBW] | PVA [PBW] | Phosphorus P [PBW] |
|---|---|---|---|---|---|---|
| G.G.S.75 | 75 | 20 | 5 | 0 | 0 | 0 |
| G.G.C. 75 | 75 | 20 | 0 | 5 | 0 | 0 |
| G.G.PVA.75 | 75 | 20 | 0 | 0 | 5 | 0 |
| G.G.P.75 | 75 | 20 | 0 | 0 | 0 | 5 |
| Other ingredients: Distilled Water—83 PBW | ||||||
| Composition | HA ± dH [°Sh] |
|---|---|
| G.G.PVA.20 | 40.14 ± 0.54 |
| G.PVA.S | 24.00 ± 2.02 |
| G.G.75 | 65.36 ± 1.13 |
| G.G.70 | 53.92 ± 4.64 |
| G.G.65 | 46.38 ± 2.56 |
| G.G.50 | 34.00 ± 1.61 |
| Compositions | Parameters | |||
|---|---|---|---|---|
| TSb [MPa] | Eb [%] | SE100 [MPa] | a0 [mm] | |
| G.G.PVA.20 | 1.20 ± 0.21 | 84.9 ± 3.7 | - | 0.46 |
| G.PVA.S | 1.12 ± 0.36 | 96.4 ± 4.7 | - | 0.65 |
| G.G.75 | 3.18 ± 0.75 | 161.0 ±2.8 | 2.51 ± 0.61 | 0.67 |
| G.G.70 | 1.86 ± 0.41 | 113.1 ± 4.6 | 2.16 ± 0.43 | 0.31 |
| G.G 65 | 1.53 ± 0.54 | 112.2 ± 4.1 | 1.96 ± 0.53 | 0.37 |
| G.G. 50 | 2.28 ± 0.27 | 118.6 ± 3.9 | 1.72 ± 0.52 | 0.57 |
| Composites | Parameters (±S.E.M.) | ||||
|---|---|---|---|---|---|
| H [°Sh] | TSb [MPa] | Eb [%] | SE100 [MPa] | a0 [mm] | |
| G.G.75—reference | 65.36 ± 1.13 | 3.18 ± 0.75 | 161.0 ± 2.8 | 2.51 ± 0.61 | 0.67 |
| G.G.S.75 | 44.92 ± 1.03 | 5.40 ± 0.31 | 15.70 ± 0.4 | 1.55± 0.80 | 0.20 |
| G.G.C. 75 | 85.38 ± 1.19 | 8.08 ± 0.57 | 139.6 ± 3.2 | 6.20 ± 0.55 | 0.21 |
| G.G.PVA.75 | 77.02 ± 1.47 | 6.71 ± 0.18 | 138.3 ± 0.5 | 5.29 ± 0.72 | 0.14 |
| G.G.P.75 | 92.26 ± 2.63 | 10.88 ± 0.45 | 75.40 ± 2.1 | 7.03± 0.65 | 0.13 |
| Composites | Tg [°C] (ONSET) | Tg [°C] (MILDPOINT) | ∆Cp [J/g∙K] |
|---|---|---|---|
| G.G.C.75 | 41.46 | 48.02 | 1.253 |
| G.G.P.75 | 35.78 | 41.54 | 1.113 |
| Composites | Polar Component [mJ/m2] | Dispersion Component [mJ/m2] | Surface Energy gS [mJ/m2] |
|---|---|---|---|
| G.G.75—reference | 5.23 | 43.34 | 48.57 |
| G.G.S.75 | 1.01 | 24.19 | 25.20 |
| G.G.C.75 | 3.02 | 41.77 | 44.79 |
| G.G.PVA.75 | 5.05 | 40.14 | 46.20 |
| G.G.P.75 | 3.89 | 49.80 | 53.69 |
| Composition | Qw | αc |
|---|---|---|
| G.G.S.75 | 40.56 ÷ 0.03 | 5.53 ÷ 0.05 |
| G.G.C. 75 | 35.45 ÷ 0.10 | 5.82 ÷ 0.04 |
| G.G.PVA.75 | 28.03 ÷ 0.08 | 8.30 ÷ 0.09 |
| G.G.P.75 | 25.11 ÷ 0.15 | 9.67 ÷ 0.05 |
| G.G.C.75 | G.G.P.75 | |||
|---|---|---|---|---|
| Element | Mass [%] | Atom [%] | Mass [%] | Atom [%] |
| Carbon | 48.48 | 54.67 | 54.58 | 61.75 |
| Oxygen | 34.04 | 28.82 | 27.01 | 22.94 |
| Phosphorus | 0.49 | 0.21 | 4.48 | 1.97 |
| Nitrogen | 16.73 | 16.18 | 0.32 | 0.14 |
| Sulfur | 0.26 | 0.11 | 13.62 | 13.21 |
| Composites | Before Aging | After Aging |
|---|---|---|
| HA ± dH [°Sh] | ||
| G.G.75—reference | 65.36 ± 1.13 | 73.18 ± 2.15 |
| G.G.S.75 | 44.92 ± 1.03 | 51.16 ± 1.94 |
| G.G.C. 75 | 85.38 ± 1.19 | 99.01 ± 2.34 |
| G.G.PVA.75 | 77.02 ± 1.47 | - |
| G.G.P.75 | 92.26 ± 2.63 | 99.74 ± 1.85 |
| Composition | dE*ab |
|---|---|
| G.G.75—Standard | 48.86 |
| G.G.S.75 | 7.44 |
| G.G.C. 75 | 12.59 |
| G.G.P.75 | 4.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prochon, M.; Dzeikala, O. Biopolymer Composites as an Alternative to Materials for the Production of Ecological Packaging. Polymers 2021, 13, 592. https://doi.org/10.3390/polym13040592
Prochon M, Dzeikala O. Biopolymer Composites as an Alternative to Materials for the Production of Ecological Packaging. Polymers. 2021; 13(4):592. https://doi.org/10.3390/polym13040592
Chicago/Turabian StyleProchon, Miroslawa, and Oleksandra Dzeikala. 2021. "Biopolymer Composites as an Alternative to Materials for the Production of Ecological Packaging" Polymers 13, no. 4: 592. https://doi.org/10.3390/polym13040592