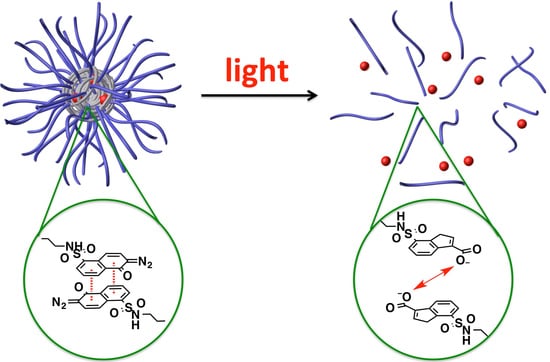
Light-Responsive Polymeric Micellar Nanoparticles with Enhanced Formulation Stability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterizations
2.3. Statistical Analysis
2.4. Synthesis of PEG-PLL-DNQ and PEG-PLL-Oct
2.5. Formulation of the Micellar Nanoparticles with DTX or Nile Red (NR)
2.6. Determination of the Critical Micelle Concentration (CMC)
2.7. Effect of Lyophilization/Reconstitution on Particle Size
2.8. Stability Studies of NPs in the Presence of BSA
2.9. Irradiation of UV Light
2.10. Determination of DTX in DTX-NPDNQ
2.11. In Vitro Cytotoxicity
2.12. Cytotoxicity of Light-Triggered DTX-NPDNQ
2.13. Intracellular Localization of the NR-NPDNQ
3. Results
3.1. Synthesis of the Light-Sensitive Block Copolymers and Formulation of the Micellar Nanoparticles
3.2. Measurements of CMC, LE and LC, and Stability
3.3. Cellular Uptake Study and Cytotoxicity
3.4. Measurement of Light-Induced Destabilization of Micellar Nanoparticles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gref, R.; Minamitake, Y.; Peracchia, M.T.; Trubetskoy, V.; Torchilin, V.; Langer, R. Biodegradable Long-Circulating Polymeric Nanospheres. Science 1994, 263, 1600–1603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, Q.; Liu, X.J.; Fu, X.Y.; Li, Q.L.; Dou, J.F.; Zhai, G.X. Current development in nanoformulations of docetaxel. Expert Opin. Drug Deliv. 2012, 9, 975–990. [Google Scholar] [CrossRef] [PubMed]
- Langer, R. Drug delivery and targeting. Nature 1998, 392, 5–10. [Google Scholar] [PubMed]
- Torchilin, V.P. Micellar nanocarriers: Pharmaceutical perspectives. Pharm. Res. 2007, 24, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Langer, R. Polymer-Controlled Drug-Delivery Systems. Acc. Chem. Res. 1993, 26, 537–542. [Google Scholar] [CrossRef]
- Hruby, M.; Konak, C.; Ulbrich, K. Polymeric micellar pH-sensitive drug delivery system for doxorubicin. J. Control. Release 2005, 103, 137–148. [Google Scholar] [CrossRef]
- Langer, R.; Folkman, J. Polymers for Sustained-Release of Proteins and Other Macromolecules. Nature 1976, 263, 797–800. [Google Scholar] [CrossRef]
- Ganta, S.; Devalapally, H.; Shahiwala, A.; Amiji, M. A review of stimuli-responsive nanocarriers for drug and gene delivery. J. Control. Release 2008, 126, 187–204. [Google Scholar] [CrossRef]
- Jain, R.K.; Stylianopoulos, T. Delivering nanomedicine to solid tumors. Nat. Rev. Clin. Oncol. 2010, 7, 653–664. [Google Scholar] [CrossRef] [Green Version]
- Carmeliet, P.; Jain, R.K. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat. Rev. Drug Discov. 2011, 10, 417–427. [Google Scholar] [CrossRef]
- Yuan, F.; Chen, Y.; Dellian, M.; Safabakhsh, N.; Ferrara, N.; Jain, R.K. Time-dependent vascular regression and permeability changes in established human tumor xenografts induced by an anti-vascular endothelial growth factor vascular permeability factor antibody. Proc. Natl. Acad. Sci. USA 1996, 93, 14765–14770. [Google Scholar] [CrossRef] [Green Version]
- Yuan, F.; Dellian, M.; Fukumura, D.; Leunig, M.; Berk, D.A.; Torchilin, V.P.; Jain, R.K. Vascular-Permeability in a Human Tumor Xenograft—Molecular-Size Dependence and Cutoff Size. Cancer Res. 1995, 55, 3752–3756. [Google Scholar] [PubMed]
- Nakayama, M.; Okano, T.; Miyazaki, T.; Kohori, F.; Sakai, K.; Yokoyama, M. Molecular design of biodegradable polymeric micelles for temperature-responsive drug release. J. Control. Release 2006, 115, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Y.; Weaver, J.V.M.; Tang, Y.Q.; Billingham, N.C.; Armes, S.P.; Tribe, K. Synthesis of shell cross-linked micelles with pH-responsive cores using ABC triblock copolymers. Macromolecules 2002, 35, 6121–6131. [Google Scholar] [CrossRef]
- Oishi, M.; Kataoka, K.; Nagasaki, Y. pH-responsive three-layered PEGylated polyplex micelle based on a lactosylated ABC triblock copolymer as a targetable and endosome-disruptive nonviral gene vector. Bioconjug. Chem. 2006, 17, 677–688. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.W.; Tong, R.; Xia, H.S.; Zhang, H.J.; Xuan, J.A. High intensity focused ultrasound and redox dual responsive polymer micelles. Chem. Commun. 2010, 46, 7739–7741. [Google Scholar] [CrossRef]
- Zhao, Y. Photocontrollable block copolymer micelles: What can we control? J. Mater. Chem. 2009, 19, 4887–4895. [Google Scholar] [CrossRef]
- Schumers, J.M.; Fustin, C.A.; Gohy, J.F. Light-responsive block copolymers. Macromol. Rapid Commun. 2010, 31, 1588–1607. [Google Scholar] [CrossRef]
- Timko, B.P.; Kohane, D.S. Prospects for near-infrared technology in remotely triggered drug delivery. Expert Opin. Drug Deliv. 2014, 11, 1681–1685. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Liu, W.; Dong, C.M. UV- and NIR-responsive polymeric nanomedicines for on-demand drug delivery. Polym. Chem. 2013, 4, 3431–3443. [Google Scholar] [CrossRef]
- Tong, R.; Chiang, H.H.; Kohane, D.S. Photoswitchable nanoparticles for in vivo cancer chemotherapy. Proc. Natl. Acad. Sci. USA 2013, 110, 19048–19053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tong, R.; Hemmati, H.D.; Langer, R.; Kohane, D.S. Photoswitchable Nanoparticles for Triggered Tissue Penetration and Drug Delivery. J. Am. Chem. Soc. 2012, 134, 8848–8855. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.A.; Liu, G.Y.; Liu, X.S.; Ji, J.A. Photo-responsive supramolecular self-assembly and disassembly of an azobenzene-containing block copolymer. Soft Matter 2010, 6, 5589–5595. [Google Scholar] [CrossRef]
- Tong, R.; Kohane, D.S. Shedding light on nanomedicine. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2012, 4, 638–662. [Google Scholar] [CrossRef] [PubMed]
- Lovell, J.F.; Liu, T.W.B.; Chen, J.; Zheng, G. Activatable Photosensitizers for Imaging and Therapy. Chem. Rev. 2010, 110, 2839–2857. [Google Scholar] [CrossRef]
- Van Dam, G.M.; Themelis, G.; Crane, L.M.A.; Harlaar, N.J.; Pleijhuis, R.G.; Kelder, W.; Sarantopoulos, A.; de Jong, J.S.; Arts, H.J.G.; van der Zee, A.G.J.; et al. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-alpha targeting: First in-human results. Nat. Med. 2011, 17, 1315–1319. [Google Scholar] [CrossRef]
- Kirmse, W. 100 years of the Wolff rearrangement. Eur. J. Org. Chem. 2002, 14, 2193–2256. [Google Scholar] [CrossRef]
- Hu, J.M.; Zhang, G.Q.; Liu, S.Y. Enzyme-responsive polymeric assemblies, nanoparticles and hydrogels. Chem. Soc. Rev. 2012, 41, 5933–5949. [Google Scholar] [CrossRef]
- Almstead, J.I.K.; Urwyler, B.; Wirz, J. Flash-Photolysis of Alpha-Diazonaphthoquinones in Aqueous-Solution—Determination of Rates and Equilibria for Keto-Enol Tautomerization of 1-Indene-3-Carboxylic Acid. J. Am. Chem. Soc. 1994, 116, 954–960. [Google Scholar] [CrossRef]
- Zhao, Y. Light-Responsive Block Copolymer Micelles. Macromolecules 2012, 45, 3647–3657. [Google Scholar] [CrossRef]
- Fomina, N.; Sankaranarayanan, J.; Almutairi, A. Photochemical mechanisms of light-triggered release from nanocarriers. Adv. Drug Del. Rev. 2012, 64, 1005–1020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotharangannagari, V.K.; Sanchez-Ferrer, A.; Ruokolainen, J.; Mezzenga, R. Photoresponsive Reversible Aggregation and Dissolution of Rod-Coil Polypeptide Diblock Copolymers. Macromolecules 2011, 44, 4569–4573. [Google Scholar] [CrossRef]
- Hu, J.M.; Liu, S.Y. Responsive Polymers for Detection and Sensing Applications: Current Status and Future Developments. Macromolecules 2010, 43, 8315–8330. [Google Scholar] [CrossRef]
- Liu, G.Y.; Chen, C.J.; Li, D.D.; Wang, S.S.; Ji, J. Near-infrared light-sensitive micelles for enhanced intracellular drug delivery. J. Mater. Chem. 2012, 22, 16865–16871. [Google Scholar] [CrossRef]
- Sun, L.; Ma, X.F.; Dong, C.M.; Zhu, B.S.; Zhu, X.Y. NIR-Responsive and Lectin-Binding Doxorubicin-Loaded Nanomedicine from Janus-Type Dendritic PAMAM Amphiphiles. Biomacromolecules 2012, 13, 3581–3591. [Google Scholar] [CrossRef]
- Goodwin, A.P.; Mynar, J.L.; Ma, Y.Z.; Fleming, G.R.; Frechet, J.M.J. Synthetic micelle sensitive to IR light via a two-photon process. J. Am. Chem. Soc. 2005, 127, 9952–9953. [Google Scholar] [CrossRef]
- Mynar, J.L.; Goodwin, A.P.; Cohen, J.A.; Ma, Y.; Fleming, G.R.; Frechet, J.M.J. Two-photon degradable supramolecular assemblies of linear-dendritic copolymers. Chem. Commun. 2007, 20, 2081–2082. [Google Scholar] [CrossRef]
- Sun, L.; Yang, Y.; Dong, C.M.; Wei, Y. Two-Photon-Sensitive and Sugar-Targeted Nanocarriers from Degradable and Dendritic Amphiphiles. Small 2011, 7, 401–406. [Google Scholar] [CrossRef]
- Attia, A.B.E.; Yang, C.; Tan, J.P.K.; Gao, S.J.; Williams, D.F.; Hedrick, J.L.; Yang, Y.Y. The effect of kinetic stability on biodistribution and anti-tumor efficacy of drug-loaded biodegradable polymeric micelles. Biomaterials 2013, 34, 3132–3140. [Google Scholar] [CrossRef]
- Kim, B.S.; Park, S.W.; Hammond, P.T. Hydrogen-bonding layer-by-layer assembled biodegradable polymeric micelles as drug delivery vehicles from surfaces. ACS Nano 2008, 2, 386–392. [Google Scholar] [CrossRef]
- Song, B.; Wang, Z.Q.; Chen, S.L.; Zhang, X.; Fu, Y.; Smet, M.; Dehaen, W. The introduction of pi-pi stacking moieties for fabricating stable micellar structure: Formation and dynamics of disklike micelles. Angew. Chem. Int. Ed. 2005, 44, 4731–4735. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Huang, Y.X.; Liu, H.; Marquez, R.T.; Lu, J.Q.; Zhao, W.C.; Zhang, X.L.; Gao, X.; Li, J.; Venkataramanan, R.; et al. A PEG-Fmoc conjugate as a nanocarrier for paclitaxel. Biomaterials 2014, 35, 7146–7156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Y.; van Steenbergen, M.J.; Teunissen, E.A.; Novo, L.; Gradmann, S.; Baldus, M.; van Nostrum, C.F.; Hennink, W.E. Pi-Pi Stacking Increases the Stability and Loading Capacity of Thermosensitive Polymeric Micelles for Chemotherapeutic Drugs. Biomacromolecules 2013, 14, 1826–1837. [Google Scholar] [CrossRef] [PubMed]
- Kang, N.; Perron, M.E.; Prud’homme, R.E.; Zhang, Y.B.; Gaucher, G.; Leroux, J.C. Stereocomplex block copolymer micelles: Core-shell nanostructures with enhanced stability. Nano Lett. 2005, 5, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, L.Q.; Wang, H.J.; Tu, K.H. Micellization phenomena of amphiphilic block copolymers based on methoxy poly(ethylene glycol) and either crystalline or amorphous poly(caprolactone-b-lactide). Biomacromolecules 2006, 7, 2492–2500. [Google Scholar] [CrossRef]
- Kim, Y.; Pourgholami, M.H.; Morris, D.L.; Stenzel, M.H. Effect of cross-linking on the performance of micelles as drug delivery carriers: A cell uptake study. Biomacromolecules 2012, 13, 814–825. [Google Scholar] [CrossRef]
- Lin, W.; He, Y.; Zhang, J.; Wang, L.; Wang, Z.; Ji, F.; Chen, S. Highly hemocompatible zwitterionic micelles stabilized by reversible cross-linkage for anti-cancer drug delivery. Colloids Surf. B Biointerfaces 2014, 115, 384–390. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Ramsey, J.D.; Samadi, A.; Atoufi, Z.; Yazdi, M.K.; Ganjali, M.R.; Amirabad, L.M.; Zangene, E.; Farokhi, M.; Formela, K.; et al. Poloxamer: A versatile tri-block copolymer for biomedical applications. Acta Biomater. 2020, 110, 37–67. [Google Scholar] [CrossRef]
- Cao, C.; Zhao, J.; Lu, M.; Garvey, C.J.; Stenzel, M.H. Correlation between Drug Loading Content and Biological Activity: The Complexity Demonstrated in Paclitaxel-Loaded Glycopolymer Micelle System. Biomacromolecules 2019, 20, 1545–1554. [Google Scholar] [CrossRef]
- Sokol, M.B.; Nikolskaya, E.D.; Yabbarov, N.G.; Zenin, V.A.; Faustova, M.R.; Belov, A.V.; Zhunina, O.A.; Mollaev, M.D.; Zabolotsky, A.I.; Tereshchenko, O.G.; et al. Development of novel PLGA nanoparticles with co-encapsulation of docetaxel and abiraterone acetate for a highly efficient delivery into tumor cells. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 1150–1158. [Google Scholar] [CrossRef]
- Oh, K.S.; Song, J.Y.; Cho, S.H.; Lee, B.S.; Kim, S.Y.; Kim, K.; Jeon, H.; Kwon, I.C.; Yuk, S.H. Paclitaxel-loaded Pluronic nanoparticles formed by a temperature-induced phase transition for cancer therapy. J. Control. Release 2010, 148, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Oh, K.S.; Yhee, J.Y.; Lee, D.E.; Kim, K.; Kwon, I.C.; Seo, J.H.; Kim, S.Y.; Yuk, S.H. Accurate sequential detection of primary tumor and metastatic lymphatics using a temperature-induced phase transition nanoparticulate system. Int. J. Nanomed. 2014, 9, 2955–2965. [Google Scholar] [CrossRef] [Green Version]
- Astafieva, I.; Zhong, X.F.; Eisenberg, A. Critical Micellization Phenomena in Block Polyelectrolyte Solutions. Macromolecules 1993, 26, 7339–7352. [Google Scholar] [CrossRef]
- Wang, W.P.; Chau, Y. Efficient and facile formation of two-component nanoparticles via aromatic moiety directed self-assembly. Chem. Commun. 2011, 47, 10224–10226. [Google Scholar] [CrossRef] [PubMed]
- Alemán-Nava, G.S.; Cuellar-Bermudez, S.P.; Cuaresma, M.; Bosma, R.; Muylaert, K.; Ritmann, B.E.; Parra, R. How to use Nile Red, a selective fluorescent stain for microalgal neutral lipids. J. Microbiol. Methods 2016, 128, 74–79. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.N.; Oh, K.S.; Shim, J.; Schlaepfer, I.R.; Karam, S.D.; Lee, J.-J. Light-Responsive Polymeric Micellar Nanoparticles with Enhanced Formulation Stability. Polymers 2021, 13, 377. https://doi.org/10.3390/polym13030377
Kim KN, Oh KS, Shim J, Schlaepfer IR, Karam SD, Lee J-J. Light-Responsive Polymeric Micellar Nanoparticles with Enhanced Formulation Stability. Polymers. 2021; 13(3):377. https://doi.org/10.3390/polym13030377
Chicago/Turabian StyleKim, Kyoung Nan, Keun Sang Oh, Jiwook Shim, Isabel R. Schlaepfer, Sana D. Karam, and Jung-Jae Lee. 2021. "Light-Responsive Polymeric Micellar Nanoparticles with Enhanced Formulation Stability" Polymers 13, no. 3: 377. https://doi.org/10.3390/polym13030377