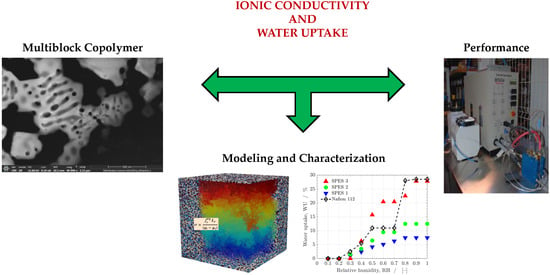
On the Conductivity of Proton-Exchange Membranes Based on Multiblock Copolymers of Sulfonated Polysulfone and Polyphenylsulfone: An Experimental and Modeling Study
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Characterization: Degree of Sulfonation, Ion-Exchange Capacity, Morphology, Water Uptake, and Ionic Conductivity
3. Numerical Model
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| area: dimensionless constant in Equation (21)/- | |
| dimensionless constant in Equation (21)/- | |
| species concentration | |
| ion-exchange capacity | |
| species diffusivity/ | |
| degree of sulfonation (i.e., percentage of sulfonated copolymer blocks)/- | |
| Faraday’s constant/ | |
| ionic current density | |
| length | |
| molecular mass | |
| number of structural units of the PPSU block/- | |
| mass of dry membrane | |
| mass of sulfonated copolymer per mol of dry sulfonated copolymer | |
| number of moles per mol of dry sulfonated copolymer/- | |
| number of sites in each spatial direction of the computational domain/- | |
| molar flux/ | |
| number of structural units of the PSU block/- | |
| unit normal vector/- | |
| universal gas constant/ | |
| membrane ionic resistance | |
| temperature | |
| ionic mobility | |
| velocity | |
| volume | |
| weight | |
| water uptake/- | |
| charge of species | |
| Greek letters | |
| average local volume fraction of water/- | |
| hydration number (number of water molecules per sulfonic group)/- | |
| density | |
| global proton conductivity | |
| bulk proton conductivity | |
| tortuosity factor/- | |
| volume fraction of water/- | |
| volume fraction of hydrated or active sites/- | |
| relative volume fraction of hydrated or active sites/- | |
| ionic potential | |
| Subscripts | |
| polymer backbone | |
| dry membrane | |
| hydrated site | |
| inlet | |
| isolated clusters | |
| outlet or bulk value | |
| sulfonated site | |
| sulfonic group | |
| total | |
| water | |
| humidified condition | |
| -direction in the material plane (first in-plane direction) | |
| thickness direction | |
| -direction in the material plane (second in-plane direction) | |
| Superscripts | |
| average | |
| threshold | |
| maximum | |
| Abbreviations | |
| FC | fuel cell |
| PEMFC | proton-exchange membrane fuel cell |
| PEM | proton-exchange membrane |
| PFSA | perfluorinated sulfonic acid |
| RH | relative humidity |
| (S)PAE | (sulfonated) poly(arylene ether) |
| PAS | poly(arylene sulfone) |
| SPESK | sulfonated poly(arylene ether sulfone ketone) |
| (S)PSU | (sulfonated) polysulfone |
| (S)PPSU | (sulfonated) polyphenylsulfone |
| FE-SEM | field emission scanning electron microscopy |
| SPES | sulfonated poly(ether sulfone) |
| SPPO | sulfonated poly(phenylene oxide) |
Appendix A


References
- Ajanovic, A.; Haas, R. Economic prospects and policy framework for hydrogen as fuel in the transport sector. Energy Policy 2018, 123, 280–288. [Google Scholar] [CrossRef]
- Hames, Y.; Kaya, K.; Baltacioglu, E.; Turksoy, A. Analysis of the control strategies for fuel saving in the hydrogen fuel cell vehicles. Int. J. Hydro. Energy 2018, 43, 1–12. [Google Scholar] [CrossRef]
- Hickner, M.A.; Ghassemi, H.; Kim, Y.S.; Einsla, B.R.; McGrath, J.E. Alternative Polymer Systems for Proton Exchange Membranes (PEMs). Chem. Rev. 2004, 104, 4587–4612. [Google Scholar] [CrossRef] [PubMed]
- Steele, B.C.H.; Heinzel, A. Materials for fuel-cell technologies. Nature 2001, 414, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Kuila, T.; Nguyen, T.X.H.; Kim, N.H.; Lau, K.-T.; Lee, J.H. Polymer membranes for high temperature proton exchange membrane fuel cell: Recent advances and challenges. Prog. Polym. Sci. 2011, 36, 813–843. [Google Scholar] [CrossRef]
- Li, N.; Guiver, M.D. Ion Transport by Nanochannels in Ion-Containing Aromatic Copolymers. Macromolecules 2014, 47, 2175–2198. [Google Scholar] [CrossRef] [Green Version]
- García-Salaberri, P.A.; Hwang, G.; Vera, M.; Weber, A.Z.; Gostick, J.T. Effective diffusivity in partially-saturated carbon-fiber gas diffusion layers: Effect of through-plane saturation distribution. Int. J. Heat Mass Transf. 2015, 86, 319–333. [Google Scholar] [CrossRef] [Green Version]
- García-Salaberri, P.A.; Gostick, J.T.; Hwang, G.; Weber, A.Z.; Vera, M. Effective diffusivity in partially-saturated carbon-fiber gas diffusion layers: Effect of local saturation and application to macroscopic continuum models. J. Power Sour. 2015, 296, 440–453. [Google Scholar] [CrossRef] [Green Version]
- García-Salaberri, P.A.; Sánchez, D.G.; Boillat, P.; Vera, M.; Friedrich, K.A. Hydration and dehydration cycles in polymer electrolyte fuel cells operated with wet anode and dry cathode feed: A neutron imaging and modeling study. J. Power Sour. 2017, 359, 634–655. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; García-Salaberri, P.A.; Zenyuk, I.V. The impact of reaction on the effective properties of multiscale catalytic porous media: A case of polymer electrolyte fuel cells. Transp. Porous Med. 2019, 128, 363–384. [Google Scholar] [CrossRef]
- Peckham, T.J.; Holdcroft, S. Structure-Morphology-Property Relationships of Non-Perfluorinated Proton-Conducting Membranes. Adv. Mater. 2010, 22, 4667–4690. [Google Scholar] [CrossRef] [PubMed]
- Jiao, K.; Li, X. Water transport in polymer electrolyte membrane fuel cells. Prog. Energy Combust. Sci. 2011, 37, 221–291. [Google Scholar] [CrossRef]
- Mauritz, K.A.; Moore, R.B. State of understanding of Nafion. Chem. Rev. 2004, 104, 4635–4685. [Google Scholar] [CrossRef] [PubMed]
- Sumner, J.J.; Creager, S.E.; Ma, J.J.; DesMarteau, D.D. Proton conductivity in Nafion® 117 and in a novel bis[(perfluoroalkyl)sulfonyl]imide ionomer membrane. J. Electrochem. Soc. 1998, 145, 107–110. [Google Scholar] [CrossRef]
- Babir, F.; Gomez, T. Efficiency and economics of proton exchange membrane (PEM) fuel cell. Int. J. Hydro. Energy 1996, 21, 891–901. [Google Scholar] [CrossRef]
- Di Noto, V.; Negro, E.; Sanchez, J.Y.; Iojoiu, C. Structure relaxation interplay of a new nanostructured membrane based on tetraethylammonium trifluoromethanesulfonate ionic liquid and neutralized Nafion 117 for high-temperature fuel cells. J. Am. Chem. Soc. 2010, 132, 2183–2195. [Google Scholar] [CrossRef]
- Park, C.H.; Lee, C.H.; Guiver, M.D.; Lee, Y.M. Sulfonated hydrocarbon membranes for medium-temperature and low-humidity proton exchange membrane fuel cells (PEMFCs). Prog. Polym. Sci. 2011, 36, 1443–1498. [Google Scholar] [CrossRef] [Green Version]
- He, G.; Li, Z.; Zhao, J.; Wang, S.; Wu, H.; Guiver, M.D.; Jiang, Z. Nanostructured lon-Exchange Membranes for Fuel Cells: Recent Advances and Perspectives. Adv. Mater. 2015, 27, 5280–5295. [Google Scholar] [CrossRef]
- Di Noto, V.; Giffin, G.A.; Vezzu, K.; Nawn, G.; Bertasi, F.; Tsai, T.; Maes, A.; Seifert, S.; Coughlin, B.; Herring, A. Interplay between solid state transitions, conductivity mechanisms, and electrical relaxations in a [PVBTMA] [Br]-b-PMB diblock copolymer membrane for electrochemical applications. Phys. Chem. Chem. Phys. 2015, 17, 31125–31139. [Google Scholar] [CrossRef]
- Elabd, Y.; Hickner, M.A. Block Copolymers for Fuel Cells. Macromolecules 2011, 44, 1–11. [Google Scholar] [CrossRef]
- Yoo, T.; Aziz, M.d.A.; Oh, K.; Shanmugam, S. Modified sulfonated Poly(arylene ether multiblock copolymers containing highly sulfonated blocks for polymer electrolyte membrane fuel cells. J. Membr. Sci. 2017, 542, 102–109. [Google Scholar] [CrossRef]
- Jung, M.S.; Kim, T.-H.; Yoon, Y.J.; Kang, C.G.; Yu, D.M.; Lee, J.Y.; Kim, H.-J.; Hong, Y.T. Sulfonated poly(arylene sulfone) multiblock copolymers for proton exchange membrane fuel cells. J. Membr. Sci. 2014, 459, 72–85. [Google Scholar] [CrossRef]
- Bae, B.; Miyatake, K.; Watanabe, M. Sulfonated poly(arylene ether sulfone ketone) multiblock copolymer with highly sulfonated block. Synthesis and properties. Macromolecules 2010, 43, 2684–2691. [Google Scholar] [CrossRef]
- Ureña, N.; Pérez Prior, M.; del Río, C.; Várez, A.; Sánchez, J.Y.; Iojoiu, C.; Levenfeld, B. Multiblock Copolymers of Sulfonated PSU/PPSU Poly(ether sulfone)s as solid electrolytes for Proton Exchange Membrane Fuel Cells. Electrochim. Acta. 2019, 301, 1–13. [Google Scholar] [CrossRef]
- Li, X.; Zhao, Y.; Wang, S.; Xie, X. Molecular dynamics simulation study of a polynorbornene-based polymer: A prediction of proton exchange membrane design and performance. Int. J. Hydrog. Energy 2016, 41, 16254–16263. [Google Scholar] [CrossRef]
- Secanell, M.; Carnes, B.; Suleman, A.; Djilali, N. Numerical optimization of proton exchange membrane fuel cell cathodes. Electrochim. Acta 2007, 52, 2668–2682. [Google Scholar] [CrossRef]
- Li, Z.; Zhong, W.; Ying, L.; Liu, F.; Li, N.; Huang, F.; Cao, Y. Morphology optimization via molecular weight tuning of donor polymer enables all-polymer solar cells with simultaneously improved performance and stability. Nano Energy 2019, 64, 103931. [Google Scholar] [CrossRef]
- Hwang, G.S.; Kaviany, M.; Gostick, J.T.; Kienitz, B.; Weber, A.Z.; Kim, M.H. Role of water states on water uptake and proton transport in Nafion using molecular simulations and bimodal network. Polymer 2011, 52, 2584–2593. [Google Scholar] [CrossRef] [Green Version]
- Allen, F.I.; Comolli, L.R.; Kusoglu, A.; Modestino, M.A.; Minor, A.M.; Weber, A.Z. Morphology of Hydrated As-Cast Nafion Revealed through Cryo Electron Tomography. ACS Macro Lett. 2015, 4, 1–5. [Google Scholar] [CrossRef]
- Kusoglu, A.; Weber, A.Z. New Insights into Perfluorinated Sulfonic-Acid Ionomers. Chem. Rev. 2017, 117, 987–1104. [Google Scholar] [CrossRef]
- Hsu, W.Y.; Barkley, J.R.; Meakin, P. Ion Percolation and Insulator-to-Conductor Transition in Nafion Perfluorosulfonic Acid Membranes. Macromolecules 1980, 13, 198–200. [Google Scholar] [CrossRef]
- Berezina, N.P.; Karpenko, L.V. Percolation Effects in Ion-Exchange Materials. Colloid. J. 2000, 62, 676–684. [Google Scholar] [CrossRef]
- Thampan, T.; Malhotra, S.; Tang, H.; Datta, R. Modeling of Conductive Transport in Proton-Exchange Membranes. J. Electrochem. Soc. 2000, 147, 3242–3250. [Google Scholar] [CrossRef]
- Costamagna, P.; Grosso, S.; Di Felice, R. Percolative model of proton conductivity of Nafion® membranes. J. Power Sour. 2008, 178, 537–546. [Google Scholar] [CrossRef]
- Karpenko-Jereb, L.; Berezina, N.P. Determination of structural, selective, electrokinetic and percolation characteristics of ion-exchange membranes from conductive data. Desalination 2009, 245, 587–596. [Google Scholar] [CrossRef]
- Berg, P.; Benjaminsen, B.E. Effects of Finite-size Ions and Relative Permittivity in a Nanopore Model of a Polymer Electrolyte Membrane. Electrochim. Acta 2014, 120, 429–438. [Google Scholar] [CrossRef]
- Eikerling, M.; Kornyshev, A.A.; Stimming, U. Electrophysical Properties of Polymer Electrolyte Membranes: A Random Network Model. J. Phys. Chem. B 1997, 101, 10807–10820. [Google Scholar] [CrossRef]
- Eikerling, M.; Kornyshev, A.A.; Kuznetsov, A.M.; Ulstrup, J.; Walbran, S. Mechanisms of Proton Conductance in Polymer Electrolyte Membranes. J. Phys. Chem. B 2001, 105, 3646–3662. [Google Scholar] [CrossRef] [Green Version]
- Weber, A.Z.; Newman, J. Transport in Polymer-Electrolyte Membranes: I. Physical Model. J. Electrochem. Soc. 2003, 150, A1008–A1015. [Google Scholar] [CrossRef]
- Weber, A.Z.; Newman, J. Transport in Polymer-Electrolyte Membranes: II. Mathematical Model. J. Electrochem. Soc. 2004, 151, A311–A325. [Google Scholar] [CrossRef]
- Tongwen, X.; Weihua, Y.; Binglin, H. Ionic conductivity threshold in sulfonated poly (phenylene oxide) matrices: A combination of three-phase model and percolation theory. Chem. Eng. Sci. 2001, 56, 5343–5350. [Google Scholar] [CrossRef]
- Gostick, J.T.; Weber, A.Z. Resistor-Network Modeling of Ionic Conduction in Polymer Electrolytes. Electrochim. Acta 2015, 179, 137–145. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Gao, H.; Xiao, C.; Tong, X.; Chen, Y. The trade-off between membrane permselectivity and conductivity: A percolation simulation of mass transport. J. Membr. Sci. 2020, 597, 117751. [Google Scholar] [CrossRef]
- Chao, S.Y.; Elsey, D.R. Process for Preparing Sulfonated poly(arylether) Resins. U.S. Patent US 4, 625, 000, 25 November 1996. [Google Scholar]
- Martos, A.M.; Sanchez, J.Y.; Varez, A.; Levenfeld, B. Electrochemical and structural characterization of sulfonated polysulfone. Polym. Test. 2015, 45, 185–193. [Google Scholar] [CrossRef] [Green Version]
- Sagar, R.U.R.; Galluzzi, M.; García-Peñas, A.; Bhat, M.A.; Zhang, M.; Staler, F.J. Large unsaturated room temperature negative magnetoresistance in graphene foam composite for wearable and flexible magnetoelectronics. Nano Res. 2018, 12, 1–7. [Google Scholar] [CrossRef]
- Sastri, V.R. High-Temperature Engineering Thermoplastics: Polysulfones, Polyimides, Polysulfides, Polyketones, Liquid Crystalline Polymers, and Fluoropolymers. In Plastics in Medical Devices. Properties, Requirements and Applications, 1st ed.; Sastri, V.R., Ed.; Elsevier: Burlington, NJ, USA, 2010; pp. 175–215. [Google Scholar]
- Li, N.; Lee, S.Y.; Liu, Y.L.; Lee, Y.M.; Guiver, M.D. A new class of highly-conducting polymer electrolyte membranes: Aromatic ABA triblock copolymers. Energy Environ. Sci. 2012, 5, 5346–5355. [Google Scholar] [CrossRef] [Green Version]
- Slade, S.M.; Ralph, T.R.; Ponce de León, C.; Campbell, S.A.; Walsh, C. The Ionic Conductivity of a Nafion® 1100 Series of Proton-exchange Membranes Re-cast from Butan-1-ol and Propan-2-ol. Fuel Cells 2010, 10, 567–574. [Google Scholar] [CrossRef] [Green Version]
- Sigwadi, R.; Dhlamini, M.S.; Mokrani, T.; Nemavhola, F.; Nonjola, P.F.; Msomi, P.F. The proton conductivity and mechanical properties of Nafion®/ZrP nanocomposite membrane. Heliyon 2019, 5, e02240. [Google Scholar] [CrossRef] [Green Version]
- Stauffer, D.; Aharony, A. Introduction to Percolation Theory, 2nd ed.; Taylor & Francis: Abingdon, UK, 2003. [Google Scholar]
- García-Salaberri, P.A. Modeling diffusion and convection in thin porous transport layers using a composite continuum-network model: Application to gas diffusion layers in polymer electrolyte fuel cells. Int. J. Heat Mass Transf. 2021, 167, 120824. [Google Scholar] [CrossRef]
- García-Salaberri, P.A.; Zenyuk, I.V.; Gostick, J.T.; Weber, A.Z. Modeling Gas Diffusion Layers in Polymer Electrolyte Fuel Cells Using a Continuum-Based Pore-Network Formulation. ECS Trans. 2020, 97, 615. [Google Scholar] [CrossRef]
- Hack, J.; García-Salaberri, P.A.; Kok, M.D.R.; Jervis, R.; Shearing, P.R.; Brandon, N.; Brett, D.J.L. X-ray Micro-Computed Tomography of Polymer Electrolyte Fuel Cells: What is the Representative Elementary Area? J. Electrochem. Soc. 2020, 167, 013545. [Google Scholar] [CrossRef]
- García-Salaberri, P.A.; Gostick, J.T.; Zenyuk, I.V.; Hwang, G.; Vera, M.; Weber, A.Z. On the limitations of volume-averaged descriptions of gas diffusion layers in the modeling of polymer electrolyte fuel cells. ECS Trans. 2017, 80, 133. [Google Scholar] [CrossRef]
- Agnaou, M.; Sadeghi, M.A.; Tranter, T.G.; Gostick, J.T. Modeling transport of charged species in pore networks: Solution of the Nernst–Planck equations coupled with fluid flow and charge conservation equations. Comput. Geosci. 2020, 140, 104505. [Google Scholar] [CrossRef]
- Schmidt, S.A. Mathematical Models of Ion Transport through Nafion Membranes in Modified Electrodes and Fuel Cells without Electroneutrality. Ph.D. Thesis, University of Iowa, Iowa City, IA, USA, 2010. [Google Scholar]
- Kim, Y.S.; Lee, K.S. Fuel Cell Membrane Characterizations. Polym. Rev. 2015, 55, 330–370. [Google Scholar] [CrossRef]
- Li, N.; Hwang, D.S.; Lee, S.Y.; Liu, Y.L.; Lee, Y.M.; Guiver, M.D. Densely Sulfophenylated Segmented Copoly(arylene ether sulfone). Macromolecules 2011, 44, 4901–4910. [Google Scholar] [CrossRef] [Green Version]
- Sel, O.; Thi Kim, L.T.; Debiemme-Chouvy, C.; Gabrielli, C.; Laberty-Robert, C.; Perrot, H. Determination of the Diffusion Coefficient of Protons in Nafion Thin Films by ac-Electrogravimetry. Langmuir 2013, 29, 13655–13660. [Google Scholar] [CrossRef]
- Fimrite, J.; Struchtrup, H.; Djilali, N. Transport Phenomena in Polymer Electrolyte Membranes: I. Modeling Framework. J. Electrochem. Soc. 2005, 152, A1804–A1814. [Google Scholar] [CrossRef]
- Kreuer, K.D. On the development of proton conducting materials for technological applications. Solid State Ion. 1997, 97, 1–15. [Google Scholar] [CrossRef]
- Lapid, H.; Agmon, N.; Petersen, M.K.; Voth, G.A. A bond-order analysis of the mechanism for hydrated proton mobility in liquid water. J. Chem. Phys. 2005, 122, 014506. [Google Scholar] [CrossRef] [Green Version]
- Feng, S.; Voth, G.A. Proton Solvation and Transport in Hydrated Nafion. J. Phys. Chem. B 2011, 115, 5903–5912. [Google Scholar] [CrossRef]
- Petersen, M.K.; Voth, G.A. Characterization of the Solvation and Transport of the Hydrated Proton in the Perfluorosulfonic Acid Membrane Nafion. J. Phys. Chem. B 2006, 110, 18594–18600. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.K.; Hatt, A.J.; Voth, G.A. Orientational Dynamics of Water in the Nafion Polymer Electrolyte Membrane and Its Relationship to Proton Transport. J. Phys. Chem. B 2008, 112, 7754–7761. [Google Scholar] [CrossRef] [PubMed]
- Selvan, M.E.; Keffer, D.J.; Cui, S.; Paddison, S.J. A Reactive Molecular Dynamics Algorithm for Proton Transport in Aqueous Systems. J. Phys. Chem. C 2010, 114, 11965–11976. [Google Scholar] [CrossRef]
- Mitescu, C.D.; Musolf, M.J. Critical exponent for 3-D percolation conductivity, revisited. J. Phys. Lett. 1983, 44, 679–683. [Google Scholar] [CrossRef]
- Kim, Y.; Ketpang, K.; Jaritphun, S.; Park, J.S.; Shanmugam, S. A polyoxometalate coupled graphene oxide–Nafion composite membrane for fuel cells operating at low relative humidity. J. Mater. Chem. A 2015, 3, 8148–8155. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Lee, S.Y.; Shin, D.W.; Kang, N.R.; Lee, Y.M.; Guiver, M.D. Proton-conducting membranes from poly(ethersulfone)s grafted with sulfoalkylamine. J. Membr. Sci. 2013, 427, 443–450. [Google Scholar] [CrossRef] [Green Version]
- Ahn, M.K.; Lee, S.B.; Min, C.M.; Yu, Y.G.; Jang, J.; Gim, M.Y.; Lee, J.S. Enhanced Proton Conductivity at Low Humidity of Proton Exchange Membranes with Triazole Moieties in the Side Chains. J. Membr. Sci. 2017, 523, 480–486. [Google Scholar] [CrossRef]
- Fernandes, A.C.; Ticianelli, E.D. A performance and degradation study of Nafion 212 membrane for proton exchange membrane fuel cells. J. Power Sour. 2009, 193, 547–554. [Google Scholar] [CrossRef]
- Li, G.; Pickup, P.G. Ionic Conductivity of PEMFC Electrodes. Effect of Nafion Loading. J. Electrochem. Soc. 2003, 150, C745–C752. [Google Scholar] [CrossRef]
- Suzuki, A.; Sen, U.; Hattori, T.; Miura, R.; Nagumo, R.; Tsuboi, H.; Hatakeyama, N.; Endou, A.; Takaba, H.; Williams, M.C.; et al. Ionomer content in the catalyst layer of polymer electrolyte membrane fuel cell (PEMFC): Effects on diffusion and performance. Int, J. Hydrog. Energy 2011, 36, 2221–2229. [Google Scholar] [CrossRef]
- Assumma, L.; Nguyen, H.D.; Iojoiu, C.; Lyonnard, S.; Mercier, R.; Espuche, E. Sulfonated poly(arylenesulfone) multiblock copolymers for proton exchange membrane fuel cells. ACS Appl. Mater. Interfaces 2015, 7, 13808–13820. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.D.; Assumma, L.; Judeinstein, P.; Mercier, R.; Porcar, L.; Jestin, J.; Iojoiu, C.; Lyonnard, S. Controlling Microstructure−Transport Interplay in Highly Phase-Separated Perfluorosulfonated Aromatic Multiblock Ionomers via Molecular Architecture Design. ACS Appl. Mater. Interfaces 2017, 9, 1671–1683. [Google Scholar] [CrossRef] [PubMed]
- Balog, S.; Gasser, U.; Mortensen, K.; Gubler, L.; Scherer, G.G.; Ben Youcef, H. Correlation between Morphology, Water Uptake, and Proton Conductivity in Radiation-Grafted Proton-Exchange Membranes. Macromol. Chem. Phys. 2010, 211, 635–643. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, K.E.; Shafique, N.; Douglas, J.F.; Starr, F. Cooperative dynamics in a model DPPC membrane arise from membrane layer interactions. Emerg. Mater. 2019, 2, 1–10. [Google Scholar] [CrossRef]
- Hayirlioglu, A.; Kulkarni, M.; Singh, G.; Al-Enizi, A.M.; Zvonkina, I.; Alamgir, K. Block copolymer ordering on elastomeric substrates of tunable surface energy. Emerg. Mater. 2019, 2, 11–22. [Google Scholar] [CrossRef] [Green Version]
- Elgawady, Y.; Ponnamma, D.; Adham, S.; Al-Maas, M.; Ammar, A.; Alamgir, K.; Al-Maadeed, M.A.; Hassan, M.K. Mesoporous silica filled smart super oleophilic fibers of triblock copolymer nanocomposites for oil absorption applications. Emergent Mater. 2020, 3, 279–290. [Google Scholar] [CrossRef]
- Kusoglu, A.; Vezzu, K.; Hegde, G.A.; Nawn, G.; Motz, A.R.; Sarode, H.N.; Haugen, G.M.; Yang, Y.; Seifert, S.; Yandrasits, M.A.; et al. Transport and Morphology of a Proton Exchange Membrane Based on a Doubly Functionalized Perfluorosulfonic Imide Side Chain Perflourinated Polymer. Chem. Mater. 2020, 32, 38–59. [Google Scholar] [CrossRef]
- Li, J.; Tang, H.; Chen, L.; Chen, R.; Pan, M.; Jiang, S.P. Highly ordered and periodic mesoporous Nafion membranes via colloidal silica mediated self-assembly for fuel cells. Chem. Commun. 2013, 49, 6537–6539. [Google Scholar] [CrossRef]
- Lu, J.; Lu, S.; Jiang, S.P. Highly ordered mesoporous Nafion membranes for fuel cells. Chem. Commun. 2011, 47, 3216–3218. [Google Scholar] [CrossRef]
- Lu, J.; Tang, H.; Xu, C.; Jiang, S.P. Nafion membranes with ordered mesoporous structure and high water retention properties for fuel cell applications. J. Mater. Chem. 2012, 22, 5810. [Google Scholar] [CrossRef]
- Liu, Y.H.; Yi, B.; Shao, Z.G.; Xing, D.; Zhang, H. Carbon Nanotubes Reinforced Nafion Composite Membrane for Fuel Cell Applications. Electrochem. Solid-State Lett. 2006, 9, A356–A359. [Google Scholar] [CrossRef]
- Klose, C.; Breitwieser, M.; Vierrath, S.; Klingele, M.; Cho, H.; Büchler, A.; Kerres, J.; Thiele, S. Electrospun sulfonated poly(ether ketone) nanofibers as proton conductive reinforcement for durable Nafion composite membranes. J. Power Sour. 2017, 361, 237–242. [Google Scholar] [CrossRef]
- Liu, Y.H.; Yi, B.; Shao, Z.G.; Wang, L.; Xing, D.; Zhang, H. Pt/CNTs-Nafion reinforced and self-humidifying composite membrane for PEMFC applications. J. Power Sour. 2007, 163, 807–813. [Google Scholar] [CrossRef]
- Kusoglu, A.; Karlsson, A.M.; Santare, M.H.; Cleghorn, S.; Johnson, W.B. Mechanical response of fuel cell membranes subjected to a hygro-thermal cycle. J. Power Sour. 2006, 161, 807–813. [Google Scholar] [CrossRef] [Green Version]
- Curtin, D.E.; Lousenberg, R.D.; Henry, T.J.; Tangeman, P.C.; Tisack, M.E. Advanced materials for improved PEMFC performance and life. J. Power Sour. 2004, 131, 41–48. [Google Scholar] [CrossRef]










| Membrane | ||||
|---|---|---|---|---|
| SPES 1 [24] | 0.97 | 0.18 | 0.75 | 0.45 |
| SPES 2 [24] | 1.46 | 0.61 | 0.81 | 0.70 |
| SPES 3 [24] | 1.62 | 0.82 | 0.76 | 0.79 |
| Nafion 112 [48,49] | 0.90, 0.98 | - | - | - |
| Nafion 117 [50] | 0.93 | - | - | - |
| RH% | SPES 1 % | SPES 2 % | SPES 3 % | Nafion 112 | |||
|---|---|---|---|---|---|---|---|
| 10 | 0 | - | 0 | - | 0 | - | 0 |
| 20 | 0 | - | 0 | - | 0 | - | 0 |
| 30 | 0.9 | 0.01 | 1.5 | 0.6 | 0 | 1.53 | 2.5 |
| 40 | 2.4 | 0.03 | 3.5 | 2.19 | 6.3 | 6.6 | 5.5 |
| 50 | 4.2 | 0.18 | 6.5 | 5.73 | 15.75 | 5.64 | 11.0 |
| 60 | 5.1 | 0.51 | 9.5 | 13.02 | 20.48 | 11.4 | 11.0 |
| 70 | 6.3 | 1.02 | 9.5 | 20.97 | 20.48 | 21.75 | 11.0 |
| 80 | 7.5 | 1.53 | 12.5 | 31.86 | 22.57 | 36.63 | 28 |
| 90 (95) | 7.5 | (3.09) | 12.5 | (45.24) | 27.82 | (77.2) | 28.5 |
| 100 | 7.5 | - | 12.5 | - | 27.82 | - | 28.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ureña, N.; Pérez-Prior, M.T.; Levenfeld, B.; García-Salaberri, P.A. On the Conductivity of Proton-Exchange Membranes Based on Multiblock Copolymers of Sulfonated Polysulfone and Polyphenylsulfone: An Experimental and Modeling Study. Polymers 2021, 13, 363. https://doi.org/10.3390/polym13030363
Ureña N, Pérez-Prior MT, Levenfeld B, García-Salaberri PA. On the Conductivity of Proton-Exchange Membranes Based on Multiblock Copolymers of Sulfonated Polysulfone and Polyphenylsulfone: An Experimental and Modeling Study. Polymers. 2021; 13(3):363. https://doi.org/10.3390/polym13030363
Chicago/Turabian StyleUreña, Nieves, M. Teresa Pérez-Prior, Belén Levenfeld, and Pablo A. García-Salaberri. 2021. "On the Conductivity of Proton-Exchange Membranes Based on Multiblock Copolymers of Sulfonated Polysulfone and Polyphenylsulfone: An Experimental and Modeling Study" Polymers 13, no. 3: 363. https://doi.org/10.3390/polym13030363