Advances in Functional Biopolymer-Based Nanocomposites for Active Food Packaging Applications
Abstract
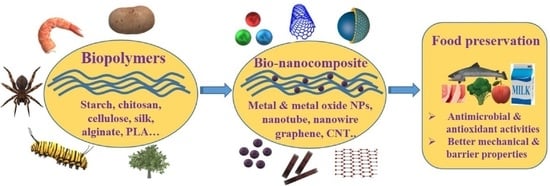
:1. Introduction
2. Polymer Nanocomposites
2.1. Classification of Polymer Nanocomposites
2.1.1. Classification Based on the Dimension of Nanofillers
2.1.2. Classification Based on the Types of Nanofillers
2.1.3. Classification Based on Type of Polymer Matrix
2.2. Methods of Polymer Nanocomposite Preparation
2.2.1. In Situ Polymerization
2.2.2. Melt Processing
2.2.3. Solution Casting
2.3. Properties of Polymer Nanocomposites
2.3.1. Mechanical Properties
2.3.2. Barrier Properties
2.3.3. Thermal Properties
2.3.4. Flame Retardancy
2.3.5. Optical Properties
3. Biodegradable Polymers (Biopolymers)
4. Applications of Bio-Based Nanocomposites for Food Packaging Systems
Active and Intelligent Packaging Systems
5. Antimicrobial Properties of Bio-Nanocomposites
Mechanisms of Antimicrobial Action
6. Conclusions and Future Trends
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Shameem, M.M.; Sasikanth, S.M.; Annamalai, R.; Raman, R.G. A brief review on polymer nanocomposites and its applications. Mater. Today Proc. 2021, 45, 2536–2539. [Google Scholar] [CrossRef]
- Sen, M. Nanocomposite materials. In Nanotechnology and the Environment; IntechOpen: London, UK, 2020. [Google Scholar]
- Liu, X.; Antonietti, M. Molten salt activation for synthesis of porous carbon nanostructures and carbon sheets. Carbon 2014, 69, 460–466. [Google Scholar] [CrossRef]
- Bustamante-Torres, M.; Romero-Fierro, D.; Arcentales-Vera, B.; Pardo, S.; Bucio, E. Interaction between Filler and Polymeric Matrix in Nanocomposites: Magnetic Approach and Applications. Polymers 2021, 13, 2998. [Google Scholar] [CrossRef]
- Guerra, D.R. Influence of Nanoparticles on the Physical Properties of Fiber Reinforced Polymer Composites. Master Thesis, The Ohio State University, Columbus, OH, USA, 2009. [Google Scholar]
- De Oliveira, A.D.; Beatrice, C.A.G. Polymer nanocomposites with different types of nanofiller. In Nanocomposites-Recent Evolution; Sivasankaran, S., Ed.; IntechOpen: London, UK, 2018; pp. 103–128. [Google Scholar]
- Abdulkadir, A.; Sarker, T.; He, Q.; Guo, Z.; Wei, S. Mössbauer spectroscopy of polymer nanocomposites. In Spectroscopy of Polymer Nanocomposites; Elsevier: Amsterdam, The Netherlands, 2016; pp. 393–409. [Google Scholar]
- Pawar, P.A.; Purwar, A.H. Biodegradable polymers in food packaging. Am. J. Eng. Res. 2013, 2, 151–164. [Google Scholar]
- Folino, A.; Karageorgiou, A.; Calabrò, P.S.; Komilis, D. Biodegradation of wasted bioplastics in natural and industrial environments: A review. Sustainability 2020, 12, 6030. [Google Scholar] [CrossRef]
- Park, S.-B.; Lih, E.; Park, K.-S.; Joung, Y.K.; Han, D.K. Biopolymer-based functional composites for medical applications. Prog. Polym. Sci. 2017, 68, 77–105. [Google Scholar] [CrossRef]
- Singh, B.G.; Das, R.P.; Kunwar, A. Protein: A versatile biopolymer for the fabrication of smart materials for drug delivery. J. Chem. Sci. 2019, 131, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Jung, E.Y.; Jin, S.K.; Hur, S.J. Analysis of the effects of biopolymer encapsulation and sodium replacement combination technology on the quality characteristics and inhibition of sodium absorption from sausage in mice. Food Chem. 2018, 250, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, N.; Gupta, S.; Gajbhiye, V.T.; Manjaiah, K.M. Optimization of isotherm models for pesticide sorption on biopolymer-nanoclay composite by error analysis. Chemosphere 2017, 173, 502–511. [Google Scholar] [CrossRef]
- Chaudhary, P.; Fatima, F.; Kumar, A. Relevance of nanomaterials in food packaging and its advanced future prospects. J. Inorg. Organomet. Polym. Mater. 2020, 30, 5180–5192. [Google Scholar] [CrossRef]
- Shankar, S.; Rhim, J.-W. Bionanocomposite films for food packaging applications. Ref. Modul. Food Sci. 2018, 1, 1–10. [Google Scholar]
- Grumezescu, A.M. Food Preservation; Academic Press: Cambridge, MA, USA, 2016; ISBN 0128043741. [Google Scholar]
- Soundararajan, N.; Borkotoky, S.S.; Katiyar, V. 7. Up-to-date advances of biobased and biodegradable polymers in food packaging. In Sustainable Polymers for Food Packaging; De Gruyter: Berlin, Germany, 2020; pp. 127–144. ISBN 3110648032. [Google Scholar]
- Skinner, G.A. Smart labelling of foods and beverages. In Advances in Food and Beverage Labelling: Information and Regulations, 1st ed.; Berryman, P., Ed.; Woodhead Publishing: Souston, UK, 2015; pp. 191–205. [Google Scholar]
- Varelis, P.; Melton, L.; Shahidi, F. Encyclopedia of Food Chemistry; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 0128140453. [Google Scholar]
- Jafarzadeh, S.; Salehabadi, A.; Nafchi, A.M.; Oladzadabbasabadi, N.; Jafari, S.M. Cheese packaging by edible coatings and biodegradable nanocomposites; improvement in shelf life, physicochemical and sensory properties. Trends Food Sci. Technol. 2021, 116, 218–231. [Google Scholar] [CrossRef]
- Primožič, M.; Knez, Ž.; Leitgeb, M. (Bio) Nanotechnology in Food Science—Food Packaging. Nanomaterials 2021, 11, 292. [Google Scholar] [CrossRef] [PubMed]
- Rhim, J.-W.; Park, H.-M.; Ha, C.-S. Bio-nanocomposites for food packaging applications. Prog. Polym. Sci. 2013, 38, 1629–1652. [Google Scholar] [CrossRef]
- Othman, S.H. Bio-nanocomposite materials for food packaging applications: Types of biopolymer and nano-sized filler. Agric. Agric. Sci. Procedia 2014, 2, 296–303. [Google Scholar] [CrossRef] [Green Version]
- Tsai, S.W.; Hahn, H.T. Introduction to Composite Materials; Routledge: Boca Raton, FL, USA, 2018; ISBN 0203750144. [Google Scholar]
- Sharma, A.K.; Bhandari, R.; Aherwar, A.; Rimašauskienė, R. Matrix materials used in composites: A comprehensive study. Mater. Today Proc. 2020, 21, 1559–1562. [Google Scholar] [CrossRef]
- Vera, M.; Mella, C.; Urbano, B.F. Smart polymer nanocomposites: Recent advances and perspectives. J. Chil. Chem. Soc. 2020, 65, 4973–4981. [Google Scholar] [CrossRef]
- Sothornvit, R. Nanostructured materials for food packaging systems: New functional properties. Curr. Opin. Food Sci. 2019, 25, 82–87. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Issaabadi, Z.; Sajjadi, M.; Sajadi, S.M.; Atarod, M. Types of nanostructures. Interface Sci. Technol. 2019, 28, 29–80. [Google Scholar]
- Gacitua, W.; Ballerini, A.; Zhang, J. Polymer nanocomposites: Synthetic and natural fillers a review. Maderas Cienc. Tecnol. 2005, 7, 159–178. [Google Scholar] [CrossRef] [Green Version]
- Vasile, C. Polymeric nanocomposites and nanocoatings for food packaging: A review. Materials 2018, 11, 1834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olivera, N.; Rouf, T.B.; Bonilla, J.C.; Carriazo, J.G.; Dianda, N.; Kokini, J.L. Effect of LAPONITE® addition on the mechanical, barrier and surface properties of novel biodegradable kafirin nanocomposite films. J. Food Eng. 2019, 245, 24–32. [Google Scholar] [CrossRef]
- Albdiry, M.T.; Yousif, B.F. Toughening of brittle polyester with functionalized halloysite nanocomposites. Compos. Part B Eng. 2019, 160, 94–109. [Google Scholar] [CrossRef]
- Lin, Y.; Hu, S.; Wu, G. Structure, dynamics, and mechanical properties of polyimide-grafted silica nanocomposites. J. Phys. Chem. C 2019, 123, 6616–6626. [Google Scholar] [CrossRef]
- Müller, K.; Bugnicourt, E.; Latorre, M.; Jorda, M.; Echegoyen Sanz, Y.; Lagaron, J.M.; Miesbauer, O.; Bianchin, A.; Hankin, S.; Bölz, U. Review on the processing and properties of polymer nanocomposites and nanocoatings and their applications in the packaging, automotive and solar energy fields. Nanomaterials 2017, 7, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaïri, F.; Gloaguen, J.-M.; Naït-Abdelaziz, M.; Mesbah, A.; Lefebvre, J.-M. Study of the effect of size and clay structural parameters on the yield and post-yield response of polymer/clay nanocomposites via a multiscale micromechanical modelling. Acta Mater. 2011, 59, 3851–3863. [Google Scholar] [CrossRef]
- Sharma, A.K.; Kaith, B.S.; Panchal, S.; Bhatia, J.K.; Bajaj, S.; Tanwar, V.; Sharma, N. Response surface methodology directed synthesis of luminescent nanocomposite hydrogel for trapping anionic dyes. J. Environ. Manag. 2019, 231, 380–390. [Google Scholar] [CrossRef]
- Lim, C.; Shin, Y.; Jung, J.; Kim, J.H.; Lee, S.; Kim, D.-H. Stretchable conductive nanocomposite based on alginate hydrogel and silver nanowires for wearable electronics. APL Mater. 2018, 7, 31502. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Ke, Y.; Zhao, Y.; Lu, S.; Deng, Q.; Yu, C.; Peng, F. Synthesis, characterization and solution properties of β-cyclodextrin-functionalized polyacrylamide/montmorillonite nanocomposites. Colloids Surf. A Physicochem. Eng. Asp. 2019, 560, 336–343. [Google Scholar] [CrossRef]
- Chatterjee, T.N.; Das, D.; Roy, R.B.; Tudu, B.; Hazarika, A.K.; Sabhapondit, S.; Tamuly, P.; Bandyopadhyay, R. Development of a nickel hydroxide nanopetal decorated molecular imprinted polymer based electrode for sensitive detection of epigallocatechin-3-gallate in green tea. Sens. Actuators B Chem. 2019, 283, 69–78. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, S.; Yang, F.; Hu, F.; Yan, B.; Gu, Y.; Jiang, H.; Cao, Y.; Xiang, M. High-performance electrochromic device based on novel polyaniline nanofibers wrapped antimony-doped tin oxide/TiO2 nanorods. Org. Electron. 2019, 65, 341–348. [Google Scholar] [CrossRef]
- Mbese, J.Z.; Ajibade, P.A. Preparation and characterization of ZnS, CdS and HgS/poly (methyl methacrylate) nanocomposites. Polymers 2014, 6, 2332–2344. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.-Y.; Leuteritz, A.; Wang, Y.-Z.; Wagenknecht, U.; Heinrich, G. Preparation and burning behaviors of flame retarding biodegradable poly (lactic acid) nanocomposite based on zinc aluminum layered double hydroxide. Polym. Degrad. Stab. 2010, 95, 2474–2480. [Google Scholar] [CrossRef]
- Zou, H.; Wu, S.; Shen, J. Polymer/silica nanocomposites: Preparation, characterization, properties, and applications. Chem. Rev. 2008, 108, 3893–3957. [Google Scholar] [CrossRef] [PubMed]
- Koo, J.H. Polymer Nanocomposites: Processing, Characterization, and Applications; McGraw-Hill Education: New York, NY, USA, 2019; ISBN 1260132315. [Google Scholar]
- Lampman, S. Characterization and Failure Analysis of Plastics; ASM International: Geauga County, OH, USA, 2003; ISBN 1615030735. [Google Scholar]
- De, S.K.; White, J.R. Rubber Technologist’s Handbook; Smithers Rapra Publishing: Shropshire, UK, 2001; Volume 1, ISBN 1859572626. [Google Scholar]
- Erceg, M.; Jozić, D.; Banovac, I.; Perinović, S.; Bernstorff, S. Preparation and characterization of melt intercalated poly (ethylene oxide)/lithium montmorillonite nanocomposites. Thermochim. Acta 2014, 579, 86–92. [Google Scholar] [CrossRef]
- Rhim, J.-W. Potential use of biopolymer-based nanocomposite films in food packaging applications. Food Sci. Biotechnol. 2007, 16, 691–709. [Google Scholar]
- Zeman, S. Mechanical properties to measure resistance of food packaging materials to external influences. Tech. Sci. 2007, 10, 35–40. [Google Scholar] [CrossRef] [Green Version]
- Sen, S.; Thomin, J.D.; Kumar, S.K.; Keblinski, P. Molecular underpinnings of the mechanical reinforcement in polymer nanocomposites. Macromolecules 2007, 40, 4059–4067. [Google Scholar] [CrossRef]
- Youssef, A.M. Polymer nanocomposites as a new trend for packaging applications. Polym. Plast. Technol. Eng. 2013, 52, 635–660. [Google Scholar] [CrossRef]
- Azizi Samir, M.A.S.; Alloin, F.; Dufresne, A. Review of recent research into cellulosic whiskers, their properties and their application in nanocomposite field. Biomacromolecules 2005, 6, 612–626. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Wilkie, C.A. Flame retardancy of polystyrene nanocomposites based on an oligomeric organically-modified clay containing phosphate. Polym. Degrad. Stab. 2003, 81, 539–550. [Google Scholar] [CrossRef] [Green Version]
- Hussain, F.; Hojjati, M.; Okamoto, M.; Gorga, R.E. Polymer-matrix nanocomposites, processing, manufacturing, and application: An overview. J. Compos. Mater. 2006, 40, 1511–1575. [Google Scholar] [CrossRef]
- Heidari, M.; Khomeiri, M.; Yousefi, H.; Rafieian, M.; Kashiri, M. Chitin nanofiber-based nanocomposites containing biodegradable polymers for food packaging applications. J. Consum. Prot. Food Saf. 2021, 16, 1–10. [Google Scholar] [CrossRef]
- Taib, M.N.A.M.; Julkapli, N.M. Dimensional stability of natural fiber-based and hybrid composites. In Mechanical and Physical Testing of Biocomposites, Fibre-Reinforced Composites and Hybrid Composites; Elsevier: Amsterdam, The Netherlands, 2019; pp. 61–79. [Google Scholar]
- Martinez-Garcia, J.C.; Serraïma-Ferrer, A.; Lopeandía-Fernández, A.; Lattuada, M.; Sapkota, J.; Rodríguez-Viejo, J. A Generalized Approach for Evaluating the Mechanical Properties of Polymer Nanocomposites Reinforced with Spherical Fillers. Nanomaterials 2021, 11, 830. [Google Scholar] [CrossRef]
- Shankar, S.; Rhim, J. Polymer nanocomposites for food packaging applications. In Functional and Physical Properties of Polymer Nanocomposites; John Wiley & Sons, Ltd.: West Sussex, UK, 2016. [Google Scholar] [CrossRef]
- Saritha, A.; Joseph, K. Barrier properties of nanocomposites. Polym. Compos. 2013, 2, 185–200. [Google Scholar]
- Pillai, S.K.; Ray, S.S. Inorganic-organic hybrid polymers for food packaging. In Functunal Polymers in Food Science: From Technology to Biology; Scrivener Publishing: Beverly, MA, USA, 2015; pp. 281–322. [Google Scholar]
- Duncan, T.V. Applications of nanotechnology in food packaging and food safety: Barrier materials, antimicrobials and sensors. J. Colloid Interface Sci. 2011, 363, 1–24. [Google Scholar] [CrossRef]
- Irshad, H.M.; Hakeem, A.S.; Raza, K.; Baroud, T.N.; Ehsan, M.A.; Ali, S.; Tahir, M.S. Design, Development and Evaluation of Thermal Properties of Polysulphone–CNT/GNP Nanocomposites. Nanomaterials 2021, 11, 2080. [Google Scholar] [CrossRef]
- Hussain, A.R.J.; Alahyari, A.A.; Eastman, S.A.; Thibaud-Erkey, C.; Johnston, S.; Sobkowicz, M.J. Review of polymers for heat exchanger applications: Factors concerning thermal conductivity. Appl. Therm. Eng. 2017, 113, 1118–1127. [Google Scholar] [CrossRef] [Green Version]
- Yoon, P.J.; Fornes, T.D.; Paul, D.R. Thermal expansion behavior of nylon 6 nanocomposites. Polymer 2002, 43, 6727–6741. [Google Scholar] [CrossRef]
- Jineesh, A.G.; Mohapatra, S. Thermal properties of polymer–carbon nanocomposites. In Carbon-Containing Polymer Composites; Springer: Berlin/Heidelberg, Germany, 2019; pp. 235–270. [Google Scholar]
- Xue, Y.; Guo, Y.; Rafailovich, M.H. Flame retardant polymer nanocomposites and interfaces. In Flame Retardants; Zafar, F., Ed.; IntechOpen: London, UK, 2019; pp. 41–62. [Google Scholar]
- Arao, Y. Flame retardancy of polymer nanocomposite. In Flame Retardants; Springer International Publishing: Cham, Switzerland, 2015; pp. 15–44. [Google Scholar]
- Kashiwagi, T.; Du, F.; Douglas, J.F.; Winey, K.I.; Harris, R.H.; Shields, J.R. Nanoparticle networks reduce the flammability of polymer nanocomposites. Nat. Mater. 2005, 4, 928–933. [Google Scholar] [CrossRef] [PubMed]
- Zanetti, M.; Kashiwagi, T.; Falqui, L.; Camino, G. Cone calorimeter combustion and gasification studies of polymer layered silicate nanocomposites. Chem. Mater. 2002, 14, 881–887. [Google Scholar] [CrossRef]
- Kreibig, U.; Vollmer, M. Optical Properties of Metal Clusters; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013; Volume 25, ISBN 3662091097. [Google Scholar]
- Sakhno, O.; Yezhov, P.; Hryn, V.; Rudenko, V.; Smirnova, T. Optical and nonlinear properties of photonic polymer nanocomposites and holographic gratings modified with noble metal nanoparticles. Polymers 2020, 12, 480. [Google Scholar] [CrossRef] [Green Version]
- Ponyavina, A.N.; Kachan, S.M. Plasmonic spectroscopy of 2D densely packed and layered metallic nanostructures. In Polarimetric Detection, Characterization and Remote Sensing; Springer: Berlin/Heidelberg, Germany, 2011; pp. 383–408. [Google Scholar]
- Ahmed, S. Bio-Based Materials for Food Packaging: Green and Sustainable Advanced Packaging Materials; Springer: Berlin/Heidelberg, Germany, 2018; ISBN 981131909X. [Google Scholar]
- Sorrentino, A.; Gorrasi, G.; Vittoria, V. Potential perspectives of bio-nanocomposites for food packaging applications. Trends Food Sci. Technol. 2007, 18, 84–95. [Google Scholar] [CrossRef]
- Bordes, P.; Pollet, E.; Avérous, L. Nano-biocomposites: Biodegradable polyester/nanoclay systems. Prog. Polym. Sci. 2009, 34, 125–155. [Google Scholar] [CrossRef]
- Imran, M.; Revol-Junelles, A.-M.; Martyn, A.; Tehrany, E.A.; Jacquot, M.; Linder, M.; Desobry, S. Active food packaging evolution: Transformation from micro-to nanotechnology. Crit. Rev. Food Sci. Nutr. 2010, 50, 799–821. [Google Scholar] [CrossRef] [PubMed]
- Koh, H.C.; Park, J.S.; Jeong, M.A.; Hwang, H.Y.; Hong, Y.T.; Ha, S.Y.; Nam, S.Y. Preparation and gas permeation properties of biodegradable polymer/layered silicate nanocomposite membranes. Desalination 2008, 233, 201–209. [Google Scholar] [CrossRef]
- Petersson, L.; Kvien, I.; Oksman, K. Structure and thermal properties of poly (lactic acid)/cellulose whiskers nanocomposite materials. Compos. Sci. Technol. 2007, 67, 2535–2544. [Google Scholar] [CrossRef]
- Wu, C.; Zhu, Y.; Wu, T.; Wang, L.; Yuan, Y.I.; Chen, J.; Hu, Y.; Pang, J. Enhanced functional properties of biopolymer film incorporated with curcurmin-loaded mesoporous silica nanoparticles for food packaging. Food Chem. 2019, 288, 139–145. [Google Scholar] [CrossRef]
- Hassan, B.; Chatha, S.A.S.; Hussain, A.I.; Zia, K.M.; Akhtar, N. Recent advances on polysaccharides, lipids and protein based edible films and coatings: A review. Int. J. Biol. Macromol. 2018, 109, 1095–1107. [Google Scholar] [CrossRef] [PubMed]
- Desai, K.G.H.; Jin Park, H. Recent developments in microencapsulation of food ingredients. Dry. Technol. 2005, 23, 1361–1394. [Google Scholar] [CrossRef]
- Chakravartula, S.S.N.; Lourenço, R.V.; Balestra, F.; Bittante, A.M.Q.B.; do Amaral Sobral, P.J.; Dalla Rosa, M. Influence of pitanga (Eugenia uniflora L.) leaf extract and/or natamycin on properties of cassava starch/chitosan active films. Food Packag. Shelf Life 2020, 24, 100498. [Google Scholar] [CrossRef]
- Lu, D.R.; Xiao, C.M.; Xu, S.J. Starch-based completely biodegradable polymer materials. Express Polym. Lett. 2009, 3, 366–375. [Google Scholar] [CrossRef]
- Espitia, P.J.P.; Du, W.-X.; de Jesús Avena-Bustillos, R.; Soares, N.D.F.F.; McHugh, T.H. Edible films from pectin: Physical-mechanical and antimicrobial properties—A review. Food Hydrocoll. 2014, 35, 287–296. [Google Scholar] [CrossRef]
- Peltzer, M.; Delgado, J.F.; Salvay, A.G.; Wagner, J.R. β-Glucan, a promising polysaccharide for bio-based films developments for food contact materials and medical applications. Curr. Org. Chem. 2018, 22, 1249–1254. [Google Scholar] [CrossRef]
- Akhter, R.; Masoodi, F.A.; Wani, T.A.; Rather, S.A. Functional characterization of biopolymer based composite film: Incorporation of natural essential oils and antimicrobial agents. Int. J. Biol. Macromol. 2019, 137, 1245–1255. [Google Scholar] [CrossRef]
- Ruiz-Navajas, Y.; Viuda-Martos, M.; Sendra, E.; Perez-Alvarez, J.A.; Fernández-López, J. In vitro antibacterial and antioxidant properties of chitosan edible films incorporated with Thymus moroderi or Thymus piperella essential oils. Food Control 2013, 30, 386–392. [Google Scholar] [CrossRef]
- Katiyar, V.; Tripathi, N. Functionalizing gum arabic for adhesive and food packaging applications. Plast. Res. Online 2019. [Google Scholar] [CrossRef]
- Mostafavi, F.S.; Zaeim, D. Agar-based edible films for food packaging applications-A review. Int. J. Biol. Macromol. 2020, 159, 1165–1176. [Google Scholar] [CrossRef]
- Sanchez-García, M.D. Carrageenan polysaccharides for food packaging. In Multifunctional and Nanoreinforced Polymers for Food Packaging; Elsevier: Amsterdam, The Netherlands, 2011; pp. 594–609. [Google Scholar]
- Chen, H.; Wang, J.; Cheng, Y.; Wang, C.; Liu, H.; Bian, H.; Pan, Y.; Sun, J.; Han, W. Application of protein-based films and coatings for food packaging: A review. Polymers 2019, 11, 2039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cortés-Rodríguez, M.; Villegas-Yépez, C.; González, J.H.G.; Rodríguez, P.E.; Ortega-Toro, R. Development and evaluation of edible films based on cassava starch, whey protein, and bees wax. Heliyon 2020, 6, e04884. [Google Scholar] [CrossRef] [PubMed]
- Ramos, M.; Valdes, A.; Beltran, A.; Garrigós, M.C. Gelatin-based films and coatings for food packaging applications. Coatings 2016, 6, 41. [Google Scholar] [CrossRef] [Green Version]
- Bayer, I.S. Zein in Food Packaging. In Sustainable Food Packaging Technolology; Athanassiou, A., Ed.; WILEY-VCH Publishing: Weinheim, Germany, 2021; pp. 199–224. [Google Scholar]
- Gautam, S.; Sharma, B.; Jain, P. Green Natural Protein Isolate based composites and nanocomposites: A review. Polym. Test. 2021, 99, 106626. [Google Scholar] [CrossRef]
- Lionetto, F.; Esposito Corcione, C. Recent applications of biopolymers derived from fish industry waste in food packaging. Polymers 2021, 13, 2337. [Google Scholar] [CrossRef]
- Guillaume, C.; Pinte, J.; Gontard, N.; Gastaldi, E. Wheat gluten-coated papers for bio-based food packaging: Structure, surface and transfer properties. Food Res. Int. 2010, 43, 1395–1401. [Google Scholar] [CrossRef]
- Wu, F.; Misra, M.; Mohanty, A.K. Challenges and new opportunities on barrier performance of biodegradable polymers for sustainable packaging. Prog. Polym. Sci. 2021, 117, 101395. [Google Scholar] [CrossRef]
- Guillard, V.; Gaucel, S.; Fornaciari, C.; Angellier-Coussy, H.; Buche, P.; Gontard, N. The next generation of sustainable food packaging to preserve our environment in a circular economy context. Front. Nutr. 2018, 5, 121. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, M.S.; Sani, N.; Adamu, M.; Abubakar, M.K. Biodegradable polymers for sustainable environmental and economic development. MOJ Biorg. Org. Chem. 2018, 2, 192–194. [Google Scholar]
- Nile, S.H.; Baskar, V.; Selvaraj, D.; Nile, A.; Xiao, J.; Kai, G. Nanotechnologies in food science: Applications, recent trends, and future perspectives. Nano-Micro Lett. 2020, 12, 1–34. [Google Scholar] [CrossRef] [Green Version]
- Alfei, S.; Marengo, B.; Zuccari, G. Nanotechnology application in food packaging: A plethora of opportunities versus pending risks assessment and public concerns. Food Res. Int. 2020, 137, 109664. [Google Scholar] [CrossRef]
- Guo, F.; Aryana, S.; Han, Y.; Jiao, Y. A review of the synthesis and applications of polymer–nanoclay composites. Appl. Sci. 2018, 8, 1696. [Google Scholar] [CrossRef] [Green Version]
- Luduena, L.N.; Alvarez, V.A.; Vazquez, A. Processing and microstructure of PCL/clay nanocomposites. Mater. Sci. Eng. A 2007, 460, 121–129. [Google Scholar] [CrossRef]
- Silva, F.A.G.S.; Dourado, F.; Gama, M.; Poças, F. Nanocellulose bio-based composites for food packaging. Nanomaterials 2020, 10, 2041. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.F.; Petty, A.R.; Sazama, G.T.; Swager, T.M. Single-walled carbon nanotube/metalloporphyrin composites for the chemiresistive detection of amines and meat spoilage. Angew. Chem. Int. Ed. 2015, 54, 6554–6557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fahmy, H.M.; Eldin, R.E.S.; Serea, E.S.A.; Gomaa, N.M.; AboElmagd, G.M.; Salem, S.A.; Elsayed, Z.A.; Edrees, A.; Shams-Eldin, E.; Shalan, A.E. Advances in nanotechnology and antibacterial properties of biodegradable food packaging materials. RSC Adv. 2020, 10, 20467–20484. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A.; Muzzarelli, C. Chitosan chemistry: Relevance to the biomedical sciences. In Polysaccharides I: Structure, Characterization and Use; Springer: Berlin/Heidelberg, Germany, 2005; Volume 186, pp. 151–209. [Google Scholar]
- Soysal, Ç.; Bozkurt, H.; Dirican, E.; Güçlü, M.; Bozhüyük, E.D.; Uslu, A.E.; Kaya, S. Effect of antimicrobial packaging on physicochemical and microbial quality of chicken drumsticks. Food Control 2015, 54, 294–299. [Google Scholar] [CrossRef]
- Jagadish, K.; Shiralgi, Y.; Chandrashekar, B.N.; Dhananjaya, B.L.; Srikantaswamy, S. Ecofriendly synthesis of metal/metal oxide nanoparticles and their application in food packaging and food preservation. In Impact of Nanoscience in the Food Industry; Elsevier: Amsterdam, The Netherlands, 2018; pp. 197–216. [Google Scholar]
- Fortunati, E.; Peltzer, M.; Armentano, I.; Torre, L.; Jiménez, A.; Kenny, J.M. Effects of modified cellulose nanocrystals on the barrier and migration properties of PLA nano-biocomposites. Carbohydr. Polym. 2012, 90, 948–956. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Fortunati, E.; Dominici, F.; Giovanale, G.; Mazzaglia, A.; Balestra, G.M.; Kenny, J.M.; Puglia, D. Synergic effect of cellulose and lignin nanostructures in PLA based systems for food antibacterial packaging. Eur. Polym. J. 2016, 79, 1–12. [Google Scholar] [CrossRef]
- Horst, M.F.; Quinzani, L.M.; Failla, M.D. Rheological and barrier properties of nanocomposites of HDPE and exfoliated montmorillonite. J. Thermoplast. Compos. Mater. 2014, 27, 106–125. [Google Scholar] [CrossRef]
- Sessini, V.; Arrieta, M.P.; Kenny, J.M.; Peponi, L. Processing of edible films based on nanoreinforced gelatinized starch. Polym. Degrad. Stab. 2016, 132, 157–168. [Google Scholar] [CrossRef]
- Marras, S.I.; Kladi, K.P.; Tsivintzelis, I.; Zuburtikudis, I.; Panayiotou, C. Biodegradable polymer nanocomposites: The role of nanoclays on the thermomechanical characteristics and the electrospun fibrous structure. Acta Biomater. 2008, 4, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Yang, J.; Liu, B.; Ma, X. Preparation and characterization of glycerol plasticized-pea starch/ZnO–carboxymethylcellulose sodium nanocomposites. Bioresour. Technol. 2009, 100, 2832–2841. [Google Scholar] [CrossRef]
- Oymaci, P.; Altinkaya, S.A. Improvement of barrier and mechanical properties of whey protein isolate based food packaging films by incorporation of zein nanoparticles as a novel bionanocomposite. Food Hydrocoll. 2016, 54, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Wakai, M.; Almenar, E. Effect of the presence of montmorillonite on the solubility of whey protein isolate films in food model systems with different compositions and pH. Food Hydrocoll. 2015, 43, 612–621. [Google Scholar] [CrossRef]
- Jang, W.-S.; Rawson, I.; Grunlan, J.C. Layer-by-layer assembly of thin film oxygen barrier. Thin Solid Films 2008, 516, 4819–4825. [Google Scholar] [CrossRef]
- Svagan, A.J.; Hedenqvist, M.S.; Berglund, L. Reduced water vapour sorption in cellulose nanocomposites with starch matrix. Compos. Sci. Technol. 2009, 69, 500–506. [Google Scholar] [CrossRef]
- Sung, S.H.; Chang, Y.; Han, J. Development of polylactic acid nanocomposite films reinforced with cellulose nanocrystals derived from coffee silverskin. Carbohydr. Polym. 2017, 169, 495–503. [Google Scholar] [CrossRef]
- Hassan-Nejad, M.; Ganster, J.; Bohn, A.; Pinnow, M.; Volkert, B. Bio-based nanocomposites of cellulose acetate and nano-clay with superior mechanical properties. Macromol. Symp. 2009, 280, 123–129. [Google Scholar] [CrossRef]
- Chen, G.; Wei, M.; Chen, J.; Huang, J.; Dufresne, A.; Chang, P.R. Simultaneous reinforcing and toughening: New nanocomposites of waterborne polyurethane filled with low loading level of starch nanocrystals. Polymer 2008, 49, 1860–1870. [Google Scholar] [CrossRef]
- Velásquez-Cock, J.; Ramírez, E.; Betancourt, S.; Putaux, J.-L.; Osorio, M.; Castro, C.; Gañán, P.; Zuluaga, R. Influence of the acid type in the production of chitosan films reinforced with bacterial nanocellulose. Int. J. Biol. Macromol. 2014, 69, 208–213. [Google Scholar] [CrossRef]
- De Moura, M.R.; Aouada, F.A.; Avena-Bustillos, R.J.; McHugh, T.H.; Krochta, J.M.; Mattoso, L.H.C. Improved barrier and mechanical properties of novel hydroxypropyl methylcellulose edible films with chitosan/tripolyphosphate nanoparticles. J. Food Eng. 2009, 92, 448–453. [Google Scholar] [CrossRef]
- Qin, Y.; Zhang, S.; Yu, J.; Yang, J.; Xiong, L.; Sun, Q. Effects of chitin nano-whiskers on the antibacterial and physicochemical properties of maize starch films. Carbohydr. Polym. 2016, 147, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, T.; Abdala, A.A.; Stankovich, S.; Dikin, D.A.; Herrera-Alonso, M.; Piner, R.D.; Adamson, D.H.; Schniepp, H.C.; Chen, X.; Ruoff, R.S. Functionalized graphene sheets for polymer nanocomposites. Nat. Nanotechnol. 2008, 3, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Dobrucka, R.; Cierpiszewski, R. Active and intelligent packaging food-Research and development—A Review. Pol. J. Food Nutr. Sci. 2014, 64, 7–15. [Google Scholar] [CrossRef] [Green Version]
- Fuertes, G.; Soto, I.; Carrasco, R.; Vargas, M.; Sabattin, J.; Lagos, C. Intelligent packaging systems: Sensors and nanosensors to monitor food quality and safety. J. Sens. 2016, 2016, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Biji, K.B.; Ravishankar, C.N.; Mohan, C.O.; Gopal, T.K.S. Smart packaging systems for food applications: A review. J. Food Sci. Technol. 2015, 52, 6125–6135. [Google Scholar] [CrossRef]
- Yam, K.L.; Lee, D.S. Emerging Food Packaging Technologies: Principles and Practice; Elsevier: Amsterdam, The Netherlands, 2012; ISBN 0857095668. [Google Scholar]
- Brockgreitens, J.; Abbas, A. Responsive food packaging: Recent progress and technological prospects. Compr. Rev. Food Sci. Food Saf. 2016, 15, 3–15. [Google Scholar] [CrossRef] [Green Version]
- Ramos, Ó.L.; Pereira, R.N.; Cerqueira, M.A.; Martins, J.R.; Teixeira, J.A.; Malcata, F.X.; Vicente, A.A. Bio-based nanocomposites for food packaging and their effect in food quality and safety. In Food Packaging and Preservation; Elsevier: Amsterdam, The Netherlands, 2018; pp. 271–306. [Google Scholar]
- Taherimehr, M.; YousefniaPasha, H.; Tabatabaeekoloor, R.; Pesaranhajiabbas, E. Trends and challenges of biopolymer-based nanocomposites in food packaging. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5321–5344. [Google Scholar] [CrossRef]
- Anvar, A.A.; Ahari, H.; Ataee, M. Antimicrobial properties of food nanopackaging: A new focus on foodborne pathogens. Front. Microbiol. 2021, 12, 690706. [Google Scholar] [CrossRef] [PubMed]
- Damm, C.; Münstedt, H.; Rösch, A. The antimicrobial efficacy of polyamide 6/silver-nano-and microcomposites. Mater. Chem. Phys. 2008, 108, 61–66. [Google Scholar] [CrossRef]
- Vilela, C.; Kurek, M.; Hayouka, Z.; Röcker, B.; Yildirim, S.; Antunes, M.D.C.; Nilsen-Nygaard, J.; Pettersen, M.K.; Freire, C.S.R. A concise guide to active agents for active food packaging. Trends Food Sci. Technol. 2018, 80, 212–222. [Google Scholar] [CrossRef]
- He, Y.; Li, H.; Fei, X.; Peng, L. Carboxymethyl cellulose/cellulose nanocrystals immobilized silver nanoparticles as an effective coating to improve barrier and antibacterial properties of paper for food packaging applications. Carbohydr. Polym. 2021, 252, 117156. [Google Scholar] [CrossRef] [PubMed]
- Marrez, D.A.; Abdelhamid, A.E.; Darwesh, O.M. Eco-friendly cellulose acetate green synthesized silver nano-composite as antibacterial packaging system for food safety. Food Packag. Shelf Life 2019, 20, 100302. [Google Scholar] [CrossRef]
- Thirumurugan, A.; Ramachandran, S.; Shiamala Gowri, A. Combined effect of bacteriocin with gold nanoparticles against food spoiling bacteria-an approach for food packaging material preparation. Int. Food Res. J. 2013, 20, 1909–1912. [Google Scholar]
- Rukmanikrishnan, B.; Jo, C.; Choi, S.; Ramalingam, S.; Lee, J. Flexible ternary combination of gellan gum, sodium carboxymethyl cellulose, and silicon dioxide nanocomposites fabricated by quaternary ammonium silane: Rheological, thermal, and antimicrobial properties. ACS Omega 2020, 5, 28767–28775. [Google Scholar] [CrossRef] [PubMed]
- Al-Nabulsi, A.; Osaili, T.; Sawalha, A.; Olaimat, A.N.; Albiss, B.A.; Mehyar, G.; Ayyash, M.; Holley, R. Antimicrobial activity of chitosan coating containing ZnO nanoparticles against E. coli O157: H7 on the surface of white brined cheese. Int. J. Food Microbiol. 2020, 334, 108838. [Google Scholar] [CrossRef]
- Qin, Y.; Liu, Y.; Yuan, L.; Yong, H.; Liu, J. Preparation and characterization of antioxidant, antimicrobial and pH-sensitive films based on chitosan, silver nanoparticles and purple corn extract. Food Hydrocoll. 2019, 96, 102–111. [Google Scholar] [CrossRef]
- Da Costa Brito, S.; Bresolin, J.D.; Sivieri, K.; Ferreira, M.D. Low-density polyethylene films incorporated with silver nanoparticles to promote antimicrobial efficiency in food packaging. Food Sci. Technol. Int. 2020, 26, 353–366. [Google Scholar] [CrossRef]
- Chen, Q.-Y.; Xiao, S.-L.; Shi, S.Q.; Cai, L.-P. A One-Pot Synthesis and Characterization of Antibacterial Silver Nanoparticle–Cellulose Film. Polymers 2020, 12, 440. [Google Scholar] [CrossRef] [Green Version]
- Chowdhury, S.; Teoh, Y.L.; Ong, K.M.; Zaidi, N.S.R.; Mah, S.-K. Poly (vinyl) alcohol crosslinked composite packaging film containing gold nanoparticles on shelf life extension of banana. Food Packag. Shelf Life 2020, 24, 100463. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Effect of CuS reinforcement on the mechanical, water vapor barrier, UV-light barrier, and antibacterial properties of alginate-based composite films. Int. J. Biol. Macromol. 2020, 164, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.; Wang, L.-F.; Rhim, J.-W. Preparation and properties of carbohydrate-based composite films incorporated with CuO nanoparticles. Carbohydr. Polym. 2017, 169, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, A.; Ahmadi, P.; Sani, M.A.; Ehsani, A.; Ghanbarzadeh, B. Functional biocompatible nanocomposite films consisting of selenium and zinc oxide nanoparticles embedded in gelatin/cellulose nanofiber matrices. Int. J. Biol. Macromol. 2021, 175, 87–97. [Google Scholar] [CrossRef]
- Lan, W.; Wang, S.; Zhang, Z.; Liang, X.; Liu, X.; Zhang, J. Development of red apple pomace extract/chitosan-based films reinforced by TiO2 nanoparticles as a multifunctional packaging material. Int. J. Biol. Macromol. 2021, 168, 105–115. [Google Scholar] [CrossRef]
- Li, X.; Ren, Z.; Wang, R.; Liu, L.; Zhang, J.; Ma, F.; Khan, M.Z.H.; Zhao, D.; Liu, X. Characterization and antibacterial activity of edible films based on carboxymethyl cellulose, Dioscorea opposita mucilage, glycerol and ZnO nanoparticles. Food Chem. 2021, 349, 129208. [Google Scholar] [CrossRef] [PubMed]
- Ojha, N.; Das, N. Fabrication and characterization of biodegradable PHBV/SiO2 nanocomposite for thermo-mechanical and antibacterial applications in food packaging. IET Nanobiotechnol. 2020, 14, 785–795. [Google Scholar] [CrossRef]
- Maćkiw, E.; Mąka, Ł.; Ścieżyńska, H.; Pawlicka, M.; Dziadczyk, P.; Rżanek-Boroch, Z. The Impact of Plasma-modified Films with Sulfur Dioxide, Sodium Oxide on Food Pathogenic Microorganisms. Packag. Technol. Sci. 2015, 28, 285–292. [Google Scholar] [CrossRef]
- Wu, J.; Sun, Q.; Huang, H.; Duan, Y.; Xiao, G.; Le, T. Enhanced physico-mechanical, barrier and antifungal properties of soy protein isolate film by incorporating both plant-sourced cinnamaldehyde and facile synthesized zinc oxide nanosheets. Colloids Surf. B Biointerfaces 2019, 180, 31–38. [Google Scholar] [CrossRef]
- Amjadi, S.; Almasi, H.; Ghorbani, M.; Ramazani, S. Preparation and characterization of TiO2NPs and betanin loaded zein/sodium alginate nanofibers. Food Packag. Shelf Life 2020, 24, 100504. [Google Scholar] [CrossRef]
- Swaroop, C.; Shukla, M. Nano-magnesium oxide reinforced polylactic acid biofilms for food packaging applications. Int. J. Biol. Macromol. 2018, 113, 729–736. [Google Scholar] [CrossRef] [Green Version]
- Kousheh, S.A.; Moradi, M.; Tajik, H.; Molaei, R. Preparation of antimicrobial/ultraviolet protective bacterial nanocellulose film with carbon dots synthesized from lactic acid bacteria. Int. J. Biol. Macromol. 2020, 155, 216–225. [Google Scholar] [CrossRef]
- Bi, F.; Zhang, X.; Liu, J.; Yong, H.; Gao, L.; Liu, J. Development of antioxidant and antimicrobial packaging films based on chitosan, D-α-tocopheryl polyethylene glycol 1000 succinate and silicon dioxide nanoparticles. Food Packag. Shelf Life 2020, 24, 100503. [Google Scholar] [CrossRef]
- Dias, M.V.; de Fátima, F.S.N.; Borges, S.V.; de Sousa, M.M.; Nunes, C.A.; de Oliveira, I.R.N.; Medeiros, E.A.A. Use of allyl isothiocyanate and carbon nanotubes in an antimicrobial film to package shredded, cooked chicken meat. Food Chem. 2013, 141, 3160–3166. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, S.; Lan, W.; Qin, W. Fabrication of polylactic acid/carbon nanotubes/chitosan composite fibers by electrospinning for strawberry preservation. Int. J. Biol. Macromol. 2019, 121, 1329–1336. [Google Scholar] [CrossRef] [PubMed]
- Melendez-Rodriguez, B.; Figueroa-Lopez, K.J.; Bernardos, A.; Martínez-Máñez, R.; Cabedo, L.; Torres-Giner, S.; Lagaron, J.M. Electrospun antimicrobial films of poly (3-hydroxybutyrate-co-3-hydroxyvalerate) containing eugenol essential oil encapsulated in mesoporous silica nanoparticles. Nanomaterials 2019, 9, 227. [Google Scholar] [CrossRef] [Green Version]
- Atef, M.; Rezaei, M.; Behrooz, R. Characterization of physical, mechanical, and antibacterial properties of agar-cellulose bionanocomposite films incorporated with savory essential oil. Food Hydrocoll. 2015, 45, 150–157. [Google Scholar] [CrossRef]
- Meira, S.M.M.; Zehetmeyer, G.; Scheibel, J.M.; Werner, J.O.; Brandelli, A. Starch-halloysite nanocomposites containing nisin: Characterization and inhibition of Listeria monocytogenes in soft cheese. LWT-Food Sci. Technol. 2016, 68, 226–234. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Rezaei, M.; Zandi, M.; Farahmandghavi, F. Development of bioactive fish gelatin/chitosan nanoparticles composite films with antimicrobial properties. Food Chem. 2016, 194, 1266–1274. [Google Scholar] [CrossRef]
- Abdollahi, M.; Rezaei, M.; Farzi, G. A novel active bionanocomposite film incorporating rosemary essential oil and nanoclay into chitosan. J. Food Eng. 2012, 111, 343–350. [Google Scholar] [CrossRef]
- Otoni, C.G.; de Moura, M.R.; Aouada, F.A.; Camilloto, G.P.; Cruz, R.S.; Lorevice, M.V.; de F.F. Soares, N.; Mattoso, L.H.C. Antimicrobial and physical-mechanical properties of pectin/papaya puree/cinnamaldehyde nanoemulsion edible composite films. Food Hydrocoll. 2014, 41, 188–194. [Google Scholar] [CrossRef]
- Salehudin, M.H.; Salleh, E.; Mamat, S.N.H.; Muhamad, I.I. Starch based active packaging film reinforced with empty fruit bunch (EFB) cellulose nanofiber. Procedia Chem. 2014, 9, 23–33. [Google Scholar] [CrossRef] [Green Version]
- Wen, P.; Zhu, D.-H.; Feng, K.; Liu, F.-J.; Lou, W.-Y.; Li, N.; Zong, M.-H.; Wu, H. Fabrication of electrospun polylactic acid nanofilm incorporating cinnamon essential oil/β-cyclodextrin inclusion complex for antimicrobial packaging. Food Chem. 2016, 196, 996–1004. [Google Scholar] [CrossRef] [PubMed]
- Kanmani, P.; Rhim, J.-W. Nano and nanocomposite antimicrobial materials for food packaging applications. In Future Medicine; Future Science Ltd.: London, UK; Mokpo National University: Seoul, Korea, 2014; pp. 34–48. [Google Scholar]
- Rezaei, M.; Pirsa, S.; Chavoshizadeh, S. Photocatalytic/antimicrobial active film based on wheat gluten/ZnO nanoparticles. J. Inorg. Organomet. Polym. Mater. 2020, 30, 2654–2665. [Google Scholar] [CrossRef]
- Hahn, A.; Fuhlrott, J.; Loos, A.; Barcikowski, S. Cytotoxicity and ion release of alloy nanoparticles. J. Nanopart. Res. 2012, 14, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vega-Jiménez, A.L.; Vázquez-Olmos, A.R.; Acosta-Gío, E.; Álvarez-Pérez, M.A. In vitro antimicrobial activity evaluation of metal oxide nanoparticles. In Nanoemulsions Properties, Fabrications and Applications; IntechOpen: London, UK, 2019; pp. 1–18. [Google Scholar]






| Biopolymers | Source | Properties | Applications | Ref. |
|---|---|---|---|---|
| Cellulose | Agricultural waste | Highly crystalline, chemically and thermally stable, antimicrobial properties | Biodegradable packaging, microencapsulation | [80,81,82] |
| Starch | Potato, corn, wheat | Enhanced gas barrier and consistent with antioxidant and antimicrobial properties | Encapsulation and biodegradable packaging | [83,84] |
| Pectin | Apple pomace and citrus peels | Biodegradability, biocompatibility, edibility, and versatile physical and chemical properties | Biodegradable films for food packaging and microencapsulation | [85] |
| β-D-glucan | Oat and barley | Rheological, biocompatibility and biodegradable properties | Encapsulation matrix and for film-forming preparations | [86] |
| Chitosan | Crab, shrimp, crawfish | Moderate mechanical strength, low barrier properties, inherent antimicrobial properties | Biodegradable films, and microencapsulation | [83,87,88] |
| Gums | Acacia tree | Excellent adhesive strengths, enhanced structural, thermal and gas barrier properties | Adhesive packaging applications | [89] |
| Alginate | Marine brown algae | Low oxygen permeability, vapors, flexibility, and water solubility | Intelligent and green packaging technologies | [89] |
| Agar | Marine red algae | High transparency, permeability, thermal stability, or mechanical strength of the film | Food packaging applications | [90] |
| Carrageenan | Cell walls of seaweeds | Enhancing sensory properties, reducing moisture loss | Edible biodegradable films and coatings | [91] |
| Casein | Milk, yogurt and cheese | Biodegradability, high thermal stability, non-toxicity | Protein-based coatings and films in food packaging | [92] |
| Whey | Milk, yogurt and cheese | Excellent barrier characteristics for oxygen, oil, and aroma | Biodegradable films for food packaging | [93] |
| Gelatin | Cattle bones | Enhanced mechanical, and optical, barrier effect against gas flow | Gelatin-based coatings and films for food packaging | [94] |
| Zein | Corn protein | Good barrier properties, high compatibility | Bio-based packaging and edible coatings | [95] |
| Soy proteins | Soybeans | Remarkable gas barrier and weaker mechanical properties, better antimicrobial properties | Biodegradable films and microencapsulation | [96] |
| Collagen | Fish skin, bones, fins | Improved rheological properties, high-water absorption capacity | Smart and active packaging. | [97] |
| Wheat gluten | Wheat flour | Improved structural, surface, gas barrier, and water vapor properties | Paper coating and food packaging | [98] |
| Nanofillers | Polymer Matrix | Properties | Applications | Ref. |
|---|---|---|---|---|
| Cellulose nanocrystals | PLA | Oxygen barrier | Used as polar and non-polar simulants in food packaging materials | [112] |
| Cellulose nanocrystals | PLA | Mechanical and antimicrobial | Biocidal activity in food packaging industry | [113] |
| Organoclay | LDPE and HDPE | Rheological and barrier | Oxygen permeability of polymer decreasing slowly with increases in clay concentration | [114] |
| Starch nanocrystals | Potato starch | Mechanical and thermal | Biodegradable edible films for packaging | [115] |
| MMT | PCL | Mechanical | Biodegradable polymer nanocomposites for food packaging | [116] |
| Clay ZnO | PEA starch | Mechanical strength | Medical, agriculture, drug release, and packaging fields | [117] |
| Zein NPs | WPI (whey protein isolate) | Mechanical, water vapor barrier | Effective food packaging materials. | [118] |
| MMT | WPI | mechanical | WPI film for food packaging | [119] |
| Anionic sodium MMT | PET | Oxygen transmission rate decreased | Replacement of aluminum foil in food packaging systems | [120] |
| Cellulose whiskers | PEA starch | Tensile, thermomechanical | Biodegradable edible films for packaging | [121] |
| Cellulose nanocrystals | PLA | Mechanical and oxygen barrier | Biomaterial for food packaging systems. | [122] |
| MMT | Cellulose acetate | Mechanical | Replacing oil-based high performance plastics for food packaging | [123] |
| Starch nanocrystals | Polyurethane | Mechanical | Biomaterial for food packaging systems | [124] |
| Bacterial cellulose nanoribbons | Chitosan | Mechanical | New materials for the food packaging | [125] |
| Chitosan–tripolyphosphate NPs | Hydroxypropyl methylcellulose | Mechanical and barrier properties | Improved functionality to edible films for food packaging | [126] |
| Chitin whiskers | Starch | Mechanical, water vapor barrier | Improved properties to prolong the shelf life of packaged foods | [127] |
| Graphene | Poly(methyl methacrylate) | Heat resistant and barrier properties | Promising material for food packaging systems | [128] |
| Nanomaterials | Biopolymer | Pathogens | Applications | Ref. |
|---|---|---|---|---|
| Ag | Chitosan | E. coli, Solmonella, S. aureus | Active and intelligent food packaging | [144] |
| Ag | LDPE | E. coli, E. faecalis, S. aureus | Improved food quality and safety | [145] |
| Ag | Cellulose | E. coli, S. aureus | Potential bacterial barrier in food packaging | [146] |
| Au | PVA | E. coli | Active food packaging for banana fruits | [147] |
| CuS | Agar | E. coli, L. monocytogenes | Active food packaging | [148] |
| CuO | Agar, alginate, chitosan | E. coli, L. monocytogenes | UV-screening and food packaging | [149] |
| ZnO | Gelatin, cellulose | E. coli, L. monocytogenes, S. aureus | Active food packaging | [150] |
| TiO2 | Chitosan | E. coli, S. aureus | Active multifunctional food packaging | [151] |
| ZnO | Carboxymethyl cellulose | E. coli, S. aureus | Active food packaging | [152] |
| SiO2 | PHBV | E. coli, S. aureus | Eco-friendly, cost-effective food packaging materials. | [153] |
| SO2 | PA, PE | E. coli, L. monocytogenes, S. aureus | Active packaging for selected types of foods | [154] |
| ZnO | Soy protein isolate | Aspergillus niger | Ideal packaging matrix for food preservation | [155] |
| TiO2 | Zein, sodium alginate | E. coli, S. aureus | Improved shelf life and quality of food stuffs | [156] |
| MgO | PLA | E. coli | UV-screening and active food packaging | [157] |
| Carbon dots | Bacterial nanocellulose | E. coli, L. monocytogenes | UV-screening and forgery-proof packaging | [158] |
| SiO2 | Chitosan | E. coli, S. aureus, S. typhimurium | Active food packaging | [159] |
| CNTs | Allyl isothiocyanate | Salmonella spp. | Active packaging for shredded cooked chicken | [160] |
| MWCNTs | Chitosan, PLA | E. coli, S. aureus, B. cinerea, Rhizopus | Active packaging for fruits and vegetables | [161] |
| MSN | PHBV | E. coli, S. aureus | Interlayers or coatings for active food packaging | [162] |
| Cellulose | Agar | E. coli, L. monocytogenes, S. aureus | Active packaging for safety and shelf-life of food | [163] |
| Halloysite | Starch | C. perfringenes, S. aureus, L. monocytogenes | Active and useful barrier to control food contamination. | [164] |
| Chitosan | Fish gelatin | S. aureus, L. monocytogenes, S. enteritidis, E. coli | Greater flexible films, with decrease in water vapor permeability | [165] |
| MMT | Chitosan | L. monocytogenes, E. coli, P. putida | Antioxidant and antibacterial films for food preservation | [166] |
| Cinnamaldehyde nanoemulsions | Pectin, papaya puri | E. coli, L. monocytogenes, S. aureus | Environmentally friendly antimicrobial packaging material for food applications | [167] |
| Cellulose nanofiber | Starch | B. subtilis, E. coli | Biopolymer active food packaging | [168] |
| PLA nanofibers | PLA | E. coli, S. aureus | Effectively prolong the shelf-life of pork. | [169] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basavegowda, N.; Baek, K.-H. Advances in Functional Biopolymer-Based Nanocomposites for Active Food Packaging Applications. Polymers 2021, 13, 4198. https://doi.org/10.3390/polym13234198
Basavegowda N, Baek K-H. Advances in Functional Biopolymer-Based Nanocomposites for Active Food Packaging Applications. Polymers. 2021; 13(23):4198. https://doi.org/10.3390/polym13234198
Chicago/Turabian StyleBasavegowda, Nagaraj, and Kwang-Hyun Baek. 2021. "Advances in Functional Biopolymer-Based Nanocomposites for Active Food Packaging Applications" Polymers 13, no. 23: 4198. https://doi.org/10.3390/polym13234198