Preparation and Property of Bio-Polyimide/Halloysite Nanocomposite Based on 2,5-Furandicarboxylic Acid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Modified HNTs
2.3. Preparation of Bio-Based Diamine
2.4. Preparation of Bio-Based PI/HNTs Nanocomposite
2.5. Preparation of Bio-Based PI/HNTs Nanocomposites Films
2.6. Characterization
3. Results and Discussion
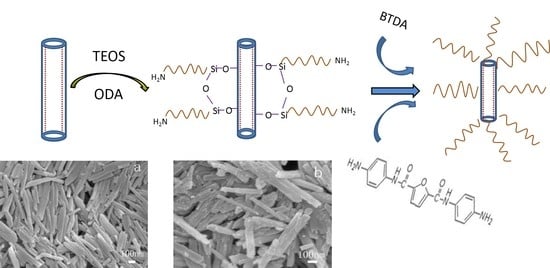
3.1. The Preparation Scheme of Bio-Based Polyimide/HNTs Films
3.2. Characterization of m-HNTs
3.3. Characterization of PI-HNTs Film
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, Y.-J.; Huang, J.-M.; Kuo, S.-W.; Lu, J.-S.; Chang, F.-C. Polyimide and polyhedral oligomeric silsesquioxane nanocomposites for low-dielectric applications. Polymer 2005, 46, 173–181. [Google Scholar] [CrossRef]
- Zhu, B.; Xie, S.; Xu, Z.; Xu, Y. Preparation and properties of the polyimide/multi-walled carbon nanotubes (MWNTs) nanocomposites. Compos. Sci. Technol. 2006, 66, 548–554. [Google Scholar] [CrossRef]
- An, L.; Pan, Y.; Shen, X.; Lu, H.; Yang, Y. Rod-like attapulgite/polyimide nanocomposites with simultaneously improved strength, toughness, thermal stability and related mechanisms. J. Mater. Chem. 2008, 18, 4928–4941. [Google Scholar] [CrossRef]
- Zhiping, S.; Hui, Z.; Yunhong, Z. Polyimides: Promising energy-storage materials. Angew. Chem. Int. Ed. 2010, 49, 8444–8448. [Google Scholar]
- Cavallaro, G.; Lazzara, G.; Milioto, S. Dispersions of Nanoclays of Different Shapes into Aqueous and Solid Biopolymeric Matrices. Extended Physicochemical Study. Langmuir ACS J. Surf. Colloids 2010, 27, 1158–1167. [Google Scholar] [CrossRef] [Green Version]
- Guo, H.; Meador, M.A.B.; McCorkle, L.; Quade, D.J.; Guo, J.; Hamilton, B.; Cakmak, M.; Sprowl, G. Polyimide Aerogels Cross-Linked through Amine Functionalized Polyoligomeric Silsesquioxane. ACS Appl. Mater. Interfaces 2011, 3, 546–552. [Google Scholar] [CrossRef]
- Luong, N.D.; Hippi, U.; Korhonen, J.; Soininen, A.J.; Ruokolainen, J.; Johansson, L.-S.; Nam, J.-D.; Sinh, L.H.; Seppälä, J. Enhanced mechanical and electrical properties of polyimide film by graphene sheets via in situ polymerization. Polymer 2011, 52, 5237–5242. [Google Scholar] [CrossRef]
- Ha, H.W.; Choudhury, A.; Kamal, T.; Kim, D.-H.; Park, S.-Y. Effect of Chemical Modification of Graphene on Mechanical, Electrical, and Thermal Properties of Polyimide/Graphene Nanocomposites. ACS Appl. Mater. Interfaces 2012, 4, 4623–4630. [Google Scholar] [CrossRef] [PubMed]
- Liaw, D.-J.; Wang, K.-L.; Huang, Y.-C.; Lee, K.-R.; Lai, J.-Y.; Ha, C.-S. Advanced polyimide materials: Syntheses, physical properties and applications. Prog. Polym. Sci. 2012, 37, 907–974. [Google Scholar] [CrossRef]
- Meador, M.A.B.; Malow, E.J.; Silva, R.; Wright, S.; Quade, D.; Vivod, S.L.; Guo, H.; Guo, J.; Cakmak, M. Mechanically Strong, Flexible Polyimide Aerogels Cross-Linked with Aromatic Triamine. ACS Appl. Mater. Interfaces 2012, 4, 536–544. [Google Scholar] [CrossRef]
- Wu, W.; Wang, K.; Zhan, M.-S. Preparation and Performance of Polyimide-Reinforced Clay Aerogel Composites. Ind. Eng. Chem. Res. 2012, 51, 12821–12826. [Google Scholar] [CrossRef]
- Chen, S.; Slattum, P.; Wang, C.; Zang, L. Self-Assembly of Perylene Imide Molecules into 1D Nanostructures: Methods, Morphologies, and Applications. Chem. Rev. 2015, 115, 11967–11998. [Google Scholar] [CrossRef] [PubMed]
- Mittal, G.; Dhand, V.; Rhee, K.Y.; Park, S.-J.; Lee, W.R. A review on carbon nanotubes and graphene as fillers in reinforced polymer nanocomposites. J. Ind. Eng. Chem. 2014, 21, 11–25. [Google Scholar] [CrossRef]
- Yao, J.; Pantano, M.F.; Pugno, N.M.; Bastiaansen, C.W.; Peijs, T. High-performance electrospun co-polyimide nanofibers. Polymer 2015, 76, 105–112. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.-B.; Wang, J.-Q.; Wang, H.-G.; Xu, Y.; Wang, Z.; Li, Z.; Mi, Y.-J.; Yang, S.-R. Preparation, mechanical and thermal properties of functionalized graphene/polyimide nanocomposites. Compos. Part A Appl. Sci. Manuf. 2012, 43, 1537–1545. [Google Scholar] [CrossRef]
- Li, Y.; He, G.; Wang, S.; Yu, S.; Pan, F.; Wu, H.; Jiang, Z. Recent advances in the fabrication of advanced composite membranes. J. Mater. Chem. A 2013, 1, 10058–10077. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, X.; Yu, S.; Jia, L.; Zhao, X.; Wang, C. Reduced Graphene Oxide-Modified Carbon Nanotube/Polyimide Film Supported MoS2Nanoparticles for Electrocatalytic Hydrogen Evolution. Adv. Funct. Mater. 2015, 25, 2693–2700. [Google Scholar] [CrossRef]
- Qiu, X.; Wang, H.; Zhou, C.; Li, D.; Liu, Y.; Yan, C. Polyimide/kaolinite composite films: Synthesis and characterization of mechanical, thermal and waterproof properties. J. Taiwan Inst. Chem. Eng. 2014, 45, 2021–2028. [Google Scholar] [CrossRef]
- Takassi, M.A.; Zadehnazari, A.; Farhadi, A.; Mallakpour, S. Highly stable polyimide composite films based on 1,2,4-triazole ring reinforced with multi-walled carbon nanotubes: Study on thermal, mechanical, and morphological properties. Prog. Org. Coat. 2015, 80, 142–149. [Google Scholar] [CrossRef]
- Fang, D.; Yao, K.; Ding, Y.; Li, P.; Hou, H. High dielectric polyimide composite film filled with a heat-resistant organic salt. Compos. Commun. 2019, 14, 29–33. [Google Scholar] [CrossRef]
- Zhao, H.; Yang, C.; Li, N.; Yin, J.; Feng, Y.; Liu, Y.; Li, J.; Li, Y.; Yue, D.; Zhu, C.; et al. Electrical and mechanical properties of polyimide composite films reinforced by ultralong titanate nanotubes. Surf. Coat. Technol. 2019, 360, 13–19. [Google Scholar] [CrossRef]
- Shin, H.I.; Chang, J.-H. Transparent Polyimide/Organoclay Nanocomposite Films Containing Different Diamine Monomers. Polymers 2020, 12, 135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danyliuk, N.; Tomaszewska, J.; Tatarchuk, T. Halloysite nanotubes and halloysite-based composites for environmental and biomedical applications. J. Mol. Liq. 2020, 309, 113077. [Google Scholar] [CrossRef]
- Cheng, C.; Song, W.; Zhao, Q.; Zhang, H. Halloysite nanotubes in polymer science: Purification, characterization, modification and applications. Nanotechnol. Rev. 2020, 9, 323–344. [Google Scholar] [CrossRef]
- Yuan, P.; Tan, D.; Annabi-Bergaya, F. Properties and applications of halloysite nanotubes: Recent research advances and future prospects. Appl. Clay Sci. 2015, 112–113, 75–93. [Google Scholar] [CrossRef]
- Bertolino, V.; Cavallaro, G.; Milioto, S.; Lazzara, G. Polysaccharides/Halloysite nanotubes for smart bionanocomposite materials. Carbohydr. Polym. 2020, 245, 116502. [Google Scholar] [CrossRef]
- Albdiry, M.; Yousif, B. Toughening of brittle polyester with functionalized halloysite nanocomposites. Compos. Part B Eng. 2018, 160, 94–109. [Google Scholar] [CrossRef]
- Hong, M.C.; Ahn, H.; Choi, M.C.; Lee, Y.; Kim, J.; Rhee, H. Pd nanoparticles immobilized on PNIPAM-halloysite: Highly active and reusable catalyst for Suzuki-Miyaura coupling reactions in water. Appl. Organomet. Chem. 2014, 28, 156–161. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, Y.; Zhou, C. Nanocomposites of halloysite and polylactide. Appl. Clay Sci. 2013, 75–76, 52–59. [Google Scholar] [CrossRef]
- Dong, Y.; Marshall, J.; Haroosh, H.J.; Mohammadzadehmoghadam, S.; Liu, D.; Qi, X.; Lau, K.-T. Polylactic acid (pla)/halloysite nanotube (hnt) composite mats: Influence of hnt content and modification. Compos. Part A Appl. Sci. Manuf. 2015, 76, 28–36. [Google Scholar] [CrossRef] [Green Version]
- Lisuzzo, L.; Cavallaro, G.; Milioto, S.; Lazzara, G. Effects of halloysite content on the thermo-mechanical performances of composite bioplastics. Appl. Clay Sci. 2019, 185, 105416. [Google Scholar] [CrossRef] [Green Version]
- Huang, D.; Zhang, Z.; Zheng, Y.; Quan, Q.; Wang, W.; Wang, A. Synergistic effect of chitosan and halloysite nanotubes on improving agar film properties. Food Hydrocoll. 2019, 101, 105471. [Google Scholar] [CrossRef]
- Yang, K.; Chi, Q.W.; Wang, X.Y.; Jiang, Y.S.; Li, F.F.; Xue, B. The role of halloy site on crystallinity, ion conductivity, thermal and mechanical properties of poly(ethylene-oxide)/halloysite nanocomposites. J. Polym. Res. 2019, 26, 138. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, Q.; Mark, J.E.; Noda, I. A novel biodegradable nanocomposite based on poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) and silylated kaolinite/silica core–shell nanoparticles. Appl. Clay Sci. 2009, 46, 51–56. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, J.; Wang, X.; Liu, B.; Yi, X. Polyimide aerogels crosslinked with MWCNT for enhanced visible-light photocatalytic activity. Appl. Surf. Sci. 2019, 478, 266–274. [Google Scholar] [CrossRef]
- Zhao, X.; Yi, X.; Wang, X.; Chu, W.; Guo, S.; Zhang, J.; Liu, B.; Liu, X. Constructing efficient polyimide(pi)/ag aerogel photocatalyst by ethanol supercritical drying technique for hydrogen evolution. Appl. Surf. Sci. 2020, 502, 144187. [Google Scholar] [CrossRef]
- Jiang, W.; Zong, X.; An, L.; Hua, S.; Miao, X.; Luan, S.; Wen, Y.; Tao, F.F.; Sun, Z. Consciously Constructing Heterojunction or Direct Z-Scheme Photocatalysts by Regulating Electron Flow Direction. ACS Catal. 2018, 8, 2209–2217. [Google Scholar] [CrossRef]
- Meng, P.; Heng, H.; Sun, Y.; Liu, X. In situ polymerization synthesis of Z-scheme tungsten trioxide/polyimide photocatalyst with enhanced visible-light photocatalytic activity. Appl. Surf. Sci. 2018, 428, 1130–1140. [Google Scholar] [CrossRef]
- Cuicui, W.; Yong, G.; Yu, Y.; Sheng, C.; Chenkun, Z.; Ying, W.; Zhigang, Z. Sulfur-doped polyimide photocatalyst with enhanced photocatalytic activity under visible light irradiation. ACS Appl. Mat. Inter. 2014, 6, 4321. [Google Scholar]
- Xiaodong, J.; Zikun, W.; Zhen, W.; Jingling, Y. Bio-based poly(ether imide)s from isohexide-derived isomeric dianhydrides. Polymers 2017, 9, 569. [Google Scholar]
- Gaili, Y.; Rui, Z.; Huahua, H.; Lixin, L.; Lei, W.; Chen, Y. Synthesis of novel biobased polyimides derived from isomannide with good optical transparency, solubility and thermal stability. RSC Adv. 2015, 5, 67574–67582. [Google Scholar]
- Hu, J.; Wang, Z.; Zheng, L.; Chang, C.; Gang, Y. Bio-based adenine-containing high performance polyimide. Polymer 2017, 119, 59–65. [Google Scholar] [CrossRef]
- Kuhire, S.S.; Sharma, P.; Chakrabarty, S.; Wadgaonkar, P.P. Partially bio-based poly(amide imide)s by polycondensation of aromatic diacylhydrazides based on lignin-derived phenolic acids and aromatic dianhydrides: Synthesis, characterization, and computational studies. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 3636–3645. [Google Scholar] [CrossRef]
- Ji, X.; Wang, Z.; Yan, J.; Wang, Z. Partially bio-based polyimides from isohexide-derived diamines. Polymer 2015, 74, 38–45. [Google Scholar] [CrossRef]
- Shin, H.; Wang, S.; Tateyama, S.; Kaneko, D.; Kaneko, T. Preparation of a Ductile Biopolyimide Film by Copolymerization. Ind. Eng. Chem. Res. 2016, 55, 8761–8766. [Google Scholar] [CrossRef]
- Suvannasara, P.; Tateyama, S.; Miyasato, A.; Matsumura, K.; Shimoda, T.; Ito, T.; Yamagata, Y.; Fujita, T.; Takaya, N.; Kaneko, T. Biobased Polyimides from 4-Aminocinnamic Acid Photodimer. Macromolecules 2014, 47, 1586–1593. [Google Scholar] [CrossRef]
- Luo, K.; Wang, Y.; Yu, J.; Zhu, J.; Hu, Z. Semi-bio-based aromatic polyamides from 2,5-furandicarboxylic acid: Toward high-performance polymers from renewable resources. RSC Adv. 2016, 6, 87013–87020. [Google Scholar] [CrossRef]
- Gomes, M.; Gandini, A.; Silvestre, A.J.D.; Reis, B. Synthesis and characterization of poly(2,5-furan dicarboxylate)s based on a variety of diols. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 3759–3768. [Google Scholar] [CrossRef]
- Knoop, R.; Vogelzang, W.; Haveren, J.V.; Es, D. High molecular weight poly(ethylene-2,5-furanoate); critical aspects in synthesis and mechanical property determination. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 4191–4199. [Google Scholar] [CrossRef]
- Ma, K.; Chen, G.; Wang, W.; Zhang, A.; Zhong, Y.; Zhang, Y.; Fang, X. Partially bio-based aromatic polyimides derived from 2,5-furandicarboxylic acid with high thermal and mechanical properties. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 1058–1066. [Google Scholar] [CrossRef]
- Barrientos-Ramírez, S.; de Oca-Ramírez, G.M.; Ramos-Fernandez, E.V.; Sepúlveda-Escribano, A.; Pastor-Blas, M.M.; González-Montiel, A. Surface modification of natural halloysite clay nanotubes with aminosilanes. Application as catalyst supports in the atom transfer radical polymerization of methyl methacrylate. Appl. Catal. A Gen. 2011, 406, 22–33. [Google Scholar] [CrossRef]
- Yuan, P.; Southon, P.D.; Liu, Z.W.; Green, M.E.R.; Hook, J.M.; Antill, S.J.; Kepert, C.J. Functionalization of halloysite clay nanotubes by grafting with gamma-aminopropyltriethoxysilane. J. Phys. Chem. C 2008, 112, 15742–15751. [Google Scholar] [CrossRef]
- Tierrablanca, E.; Romero-García, J.; Roman, P.; Cruz-Silva, R. Biomimetic polymerization of aniline using hematin supported on halloysite nanotubes. Appl. Catal. A Gen. 2010, 381, 267–273. [Google Scholar] [CrossRef]









| Samples | Mw | Mn | Mw/Mn |
|---|---|---|---|
| PI | 113,106 | 79,614 | 1.42 |
| PI-PHNTs-1% | 127,961 | 92,505 | 1.38 |
| PI-HNTs-1% | 135,636 | 98,947 | 1.37 |
| Samples | Temperature (°C) 10 wt% Loss |
|---|---|
| PI | 390.1 |
| PI-HNTs-1% | 397.1 |
| PI-PHNTs-1% | 394.9 |
| PI-HNTs-3% | 399.0 |
| PI-HNTs-5% | 403.3 |
| Samples | Peak Temperature (°C) |
|---|---|
| PI | 596.9 |
| PI-HNTs-1% | 601.3 |
| PI-HNTs-3% | 603.5 |
| PI-HNTs-5% | 608.6 |
| Sample | Tensile Strength (MPa) | Strain at Break (%) | Young’s Modulus (MPa) |
|---|---|---|---|
| PI | 79.02 | 7.410 | 1308 |
| PI-HNTs-1% | 108.85 | 9.039 | 2290 |
| PI-HNTs-3% | 97.71 | 12.460 | 1380 |
| PI-HNTs-5% | 91.30 | 8.829 | 1784 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Fan, S.; Yi, X.; Li, B.; Chen, S.; Liu, S.; Hu, T.; Chen, S. Preparation and Property of Bio-Polyimide/Halloysite Nanocomposite Based on 2,5-Furandicarboxylic Acid. Polymers 2021, 13, 4057. https://doi.org/10.3390/polym13234057
Chen Y, Fan S, Yi X, Li B, Chen S, Liu S, Hu T, Chen S. Preparation and Property of Bio-Polyimide/Halloysite Nanocomposite Based on 2,5-Furandicarboxylic Acid. Polymers. 2021; 13(23):4057. https://doi.org/10.3390/polym13234057
Chicago/Turabian StyleChen, Yingxia, Shuya Fan, Xibin Yi, Bing Li, Shiwei Chen, Shuyu Liu, Tao Hu, and Si Chen. 2021. "Preparation and Property of Bio-Polyimide/Halloysite Nanocomposite Based on 2,5-Furandicarboxylic Acid" Polymers 13, no. 23: 4057. https://doi.org/10.3390/polym13234057