Investigation on the Microscopic/Macroscopic Mechanical Properties of a Thermally Annealed Nafion® Membrane
Abstract
:1. Introduction
2. Materials and Methods
2.1. Thermal Annealing Process for a Pristine Nafion® Membrane
2.2. Measurements of Macroscopic Properties of Thermally Annealed Nafion® Membranes through Tensile Test
2.3. Measurements of Microscopic Mechanical Properties of Thermally Annealed Nafion® Membranes through AFM
2.4. Membrane Electrode Assembly (MEA) Fabrication and Fuel Cell Testing
3. Results and Discussion
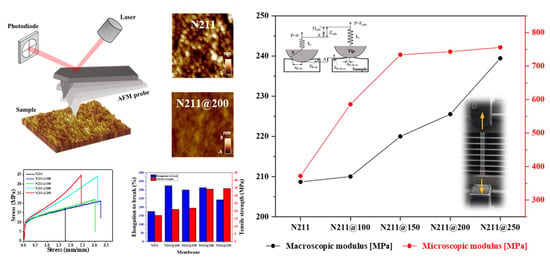
3.1. Microscopic Properties of Pristine and Thermally Annealed Nafion® Membranes
3.2. Macroscopic Mechanical Properties of Pristine and Thermally Annealed Nafion® Membranes
3.3. Relationship between Microscopic and Macroscopic Modulus
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Di Noto, V.; Lavina, S.; Negro, E.; Vittadello, M.; Conti, F.; Piga, M.; Pace, G. Hybrid inorganic-organic proton conducting membranes based on Nafion and 5 wt% of MxOy (M = Ti, Zr, Hf, Ta and W). Part II: Relaxation phenomena and conductivity mechanism. J. Power Sources 2009, 187, 57–66. [Google Scholar] [CrossRef]
- Teng, X.; Wang, M.; Li, G.; Dai, J. Polypyrrole thin film composite membrane prepared via interfacial polymerization with high selectivity for vanadium redox flow battery. React. Funct. Polym. 2020, 157, 104777. [Google Scholar] [CrossRef]
- Whiston, M.M.; Azevedo, I.L.; Litster, S.; Whitefoot, K.S.; Samaras, C.; Whitacre, J.F. Expert assessments of the cost and expected future performance of proton exchange membrane fuel cells for vehicles. Proc. Natl. Acad. Sci. USA 2019, 116, 4899–4904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edwards, P.P.; Kuznetsov, V.L.; David, W.I.F.; Brandon, N.P. Hydrogen and fuel cells: Towards a sustainable energy future. Energy Policy 2008, 36, 4356–4362. [Google Scholar] [CrossRef]
- He, K.; Zhang, C.; He, Q.; Wu, Q.; Jackson, L.; Mao, L. Effectiveness of PEMFC historical state and operating mode in PEMFC prognosis. Int. J. Hydrog. Energy 2020, 45, 32355–32366. [Google Scholar] [CrossRef]
- Kodama, K.; Nagai, T.; Kuwaki, A.; Jinnouchi, R.; Morimoto, Y. Challenges in applying highly active Pt-based nanostructured catalysts for oxygen reduction reactions to fuel cell vehicles. Nat. Nanotechnol. 2021, 16, 140–147. [Google Scholar] [CrossRef]
- Wilberforce, T.; Alaswad, A.; Palumbo, A.; Dassisti, M.; Olabi, A.G. Advances in stationary and portable fuel cell applications. Int. J. Hydrog. Energy 2016, 41, 16509–16522. [Google Scholar] [CrossRef] [Green Version]
- Saadat, N.; Dhakal, H.N.; Tjong, J.; Jaffer, S.; Yang, W.; Sain, M. Recent advances and future perspectives of carbon materials for fuel cell. Renew. Sustain. Energy Rev. 2021, 138, 110535. [Google Scholar] [CrossRef]
- Zhang, L.; Chae, S.R.; Hendren, Z.; Park, J.S.; Wiesner, M.R. Recent advances in proton exchange membranes for fuel cell applications. Chem. Eng. J. 2012, 204–206, 87–97. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, C.; Ling, C.Y.; Han, M.; Yong, R.Y.; Sun, D.; Chen, J. Review on current research of materials, fabrication and application for bipolar plate in proton exchange membrane fuel cell. Int. J. Hydrog. Energy 2020, 45, 29832–29847. [Google Scholar] [CrossRef]
- Shaari, N.; Kamarudin, S.K. Recent advances in additive-enhanced polymer electrolyte membrane properties in fuel cell applications: An overview. Int. J. Energy Res. 2019, 43, 2756–2794. [Google Scholar] [CrossRef]
- Cong Tinh, V.D.; Kim, D. Enhancement of oxidative stability of PEM fuel cell by introduction of HO• radical scavenger in Nafion ionomer. J. Memb. Sci. 2020, 613, 118517. [Google Scholar] [CrossRef]
- Pearman, B.P.; Mohajeri, N.; Brooker, R.P.; Rodgers, M.P.; Slattery, D.K.; Hampton, M.D.; Cullen, D.A.; Seal, S. The degradation mitigation effect of cerium oxide in polymer electrolyte membranes in extended fuel cell durability tests. J. Power Sources 2013, 225, 75–83. [Google Scholar] [CrossRef]
- Lee, H.; Kim, T.; Sim, W.; Kim, S.; Ahn, B.; Lim, T.; Park, K. Pinhole formation in PEMFC membrane after electrochemical degradation and wet/dry cycling test. Korean J. Chem. Eng. 2011, 28, 487–491. [Google Scholar] [CrossRef]
- De Bruijn, F.A.; Dam, V.A.T.; Janssen, G.J.M. Review: Durability and degradation issues of PEM fuel cell components. Fuel Cells 2008, 8, 3–22. [Google Scholar] [CrossRef]
- Park, Y.; Kim, D. Chemical stability enhancement of Nafion membrane by impregnation of a novel organic ·OH radical scavenger, 3,4-dihydroxy-cinnamic acid. J. Memb. Sci. 2018, 566, 1–7. [Google Scholar] [CrossRef]
- Wang, L.; Advani, S.G.; Prasad, A.K. Degradation reduction of polymer electrolyte membranes using CeO2 as a free-radical scavenger in catalyst layer. Electrochim. Acta 2013, 109, 775–780. [Google Scholar] [CrossRef]
- Coms, F.D.; Schlick, S.; Danilczuk, M. Stabilization of Perfluorinated Membranes Using Ce3+ and Mn2+ Redox Scavengers: Mechanisms and Applications. Chem. Membr. Used Fuel Cells Degrad. Stab. 2018, 75–106. [Google Scholar]
- Iwan, A.; Malinowski, M.; Pasciak, G. Polymer fuel cell components modified by graphene: Electrodes, electrolytes and bipolar plates. Renew. Sustain. Energy Rev. 2015, 49, 954–967. [Google Scholar] [CrossRef]
- He, G.; Zhao, J.; Hu, S.; Li, L.; Li, Z.; Li, Y.; Li, Z.; Wu, H.; Yang, X.; Jiang, Z. Functionalized carbon nanotube via distillation precipitation polymerization and its application in Nafion-based composite membranes. ACS Appl. Mater. Interfaces 2014, 6, 15291–15301. [Google Scholar] [CrossRef]
- Steffy, N.J.; Parthiban, V.; Sahu, A.K. Uncovering Nafion-multiwalled carbon nanotube hybrid membrane for prospective polymer electrolyte membrane fuel cell under low humidity. J. Memb. Sci. 2018, 563, 65–74. [Google Scholar] [CrossRef]
- Cao, L.; Wu, H.; Yang, P.; He, X.; Li, J.; Li, Y.; Xu, M.; Qiu, M.; Jiang, Z. Graphene oxide-based solid electrolytes with 3D percolating pathways for efficient proton transport. Adv. Funct. Mater. 2018, 28, 1804944. [Google Scholar] [CrossRef]
- Parthiban, V.; Akula, S.; Sahu, A.K. Surfactant templated nanoporous carbon-Nafion hybrid membranes for direct methanol fuel cells with reduced methanol crossover. J. Memb. Sci. 2017, 541, 127–136. [Google Scholar] [CrossRef]
- Sun, C.; Negro, E.; Nale, A.; Pagot, G.; Vezzù, K.; Zawodzinski, T.A.; Meda, L.; Gambaro, C.; Di Noto, V. An efficient barrier toward vanadium crossover in redox flow batteries: The bilayer [Nafion/(WO3)x] hybrid inorganic-organic membrane. Electrochim. Acta 2021, 378, 138133. [Google Scholar] [CrossRef]
- Narducci, R.; Knauth, P.; Chailan, J.F.; Di Vona, M.L. How to improve Nafion with tailor made annealing. RSC Adv. 2018, 8, 27268–27274. [Google Scholar] [CrossRef]
- Alberti, G.; Narducci, R.; Sganappa, M. Effects of hydrothermal/thermal treatments on the water-uptake of Nafion membranes and relations with changes of conformation, counter-elastic force and tensile modulus of the matrix. J. Power Sources 2008, 178, 575–583. [Google Scholar] [CrossRef]
- Pham, T.A.; Nam, L.V.; Choi, E.; Lee, M.S.; Jun, T.S.; Jang, S.; Kim, S.M. Mechanically Stable Thinned Membrane for a High-Performance Polymer Electrolyte Membrane Fuel Cell via a Plasma-Etching and Annealing Process. Energy Fuels 2021, 35, 11525–11532. [Google Scholar] [CrossRef]
- Li, J.; Yang, X.; Tang, H.; Pan, M. Durable and high performance Nafion membrane prepared through high-temperature annealing methodology. J. Memb. Sci. 2010, 361, 38–42. [Google Scholar] [CrossRef]
- Maldonado, L.; Perrin, J.C.; Dillet, J.; Lottin, O. Characterization of polymer electrolyte Nafion membranes: Influence of temperature, heat treatment and drying protocol on sorption and transport properties. J. Memb. Sci. 2012, 238, 43–56. [Google Scholar] [CrossRef]
- Jung, H.Y.; Kim, J.W. Role of the glass transition temperature of Nafion 117 membrane in the preparation of the membrane electrode assembly in a direct methanol fuel cell (DMFC). Int. J. Hydrog. Energy 2012, 37, 12580–12585. [Google Scholar] [CrossRef]
- Corti, H.R.; Nores-Pondal, F.; Pilar Buera, M. Low temperature thermal properties of Nafion 117 membranes in water and methanol-water mixtures. J. Power Sources 2006, 161, 799–805. [Google Scholar] [CrossRef]
- Jung, H.Y.; Cho, K.Y.; Lee, Y.M.; Park, J.K.; Choi, J.H.; Sung, Y.E. Influence of annealing of membrane electrode assembly (MEA) on performance of direct methanol fuel cell (DMFC). J. Power Sources 2007, 163, 952–956. [Google Scholar] [CrossRef]
- Iwai, Y.; Yamanishi, T. Thermal stability of ion-exchange Nafion N117CS membranes. Polym. Degrad. Stab. 2009, 94, 679–687. [Google Scholar] [CrossRef]
- Kusoglu, A.; Weber, A.Z. New Insights into Perfluorinated Sulfonic-Acid Ionomers. Chem. Rev. 2017, 117, 987–1104. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Wang, Z.; Luo, Y.; Li, J.; Zhou, Y.; Zhang, X.; Zhang, H.; Fang, P.; He, C. Thermal annealing on free volumes, crystallinity and proton conductivity of Nafion membranes. J. Phys. Chem. Solids 2018, 120, 71–78. [Google Scholar] [CrossRef]
- Lage, L.G.; Delgado, P.G.; Kawano, Y. Thermal stability and decomposition of nafion® membranes with different cations. J. Therm. Anal. Calorim. 2004, 75, 521–530. [Google Scholar] [CrossRef]
- Lee, D.H.; Oh, T.; Cho, K. Combined effect of chain length and phase state on adhesion/friction behavior of self-assembled monolayers. J. Phys. Chem. B 2005, 109, 11301–11306. [Google Scholar] [CrossRef]
- Rokni, H.; Lu, W. Direct measurements of interfacial adhesion in 2D materials and van der Waals heterostructures in ambient air. Nat. Commun. 2020, 11, 5607. [Google Scholar] [CrossRef]
- Wang, D.; Fujinami, S.; Liu, H.; Nakajima, K.; Nishi, T. Investigation of true surface morphology and nanomechanical properties of poly(styrene-b-ethylene-co-butylene-b-styrene) using nanomechanical mapping: Effects of composition. Macromolecules 2010, 43, 9049–9055. [Google Scholar] [CrossRef]
- Wang, D.; Fujinami, S.; Nakajima, K.; Nishi, T. True surface topography and nanomechanical mapping measurements on block copolymers with atomic force microscopy. Macromolecules 2010, 43, 3169–3172. [Google Scholar] [CrossRef]
- Liu, H.; Fujinami, S.; Wang, D.; Nakajima, K.; Nishi, T. Nanomechanical mapping on the deformed poly(ε-caprolactone). Macromolecules 2011, 44, 1779–1782. [Google Scholar] [CrossRef]
- Sun, Y.; Akhremitchev, B.; Walker, G.C. Using the adhesive interaction between atomic force microscopy tips and polymer surfaces to measure the elastic modulus of compliant samples. Langmuir 2004, 20, 5837–5845. [Google Scholar] [CrossRef]
- Feng, C.; Li, Y.; Qu, K.; Zhang, Z.; He, P. Mechanical behavior of a hydrated perfluorosulfonic acid membrane at meso and nano scales. RSC Adv. 2019, 9, 9594–9603. [Google Scholar] [CrossRef] [Green Version]
- Feng, M.; Qu, R.; Wei, Z.; Wang, L.; Sun, P.; Wang, Z. Characterization of the thermolysis products of Nafion membrane: A potential source of perfluorinated compounds in the environment. Sci. Rep. 2015, 5, 9859. [Google Scholar] [CrossRef] [PubMed]
- Rofifah, D. PEM Fuel Cell Properties of Pre-Stretched Recast Nafion®. Pap. Knowl. Towar. Media Hist. Doc. 2020, 16, 12–26. [Google Scholar]
- Tang, Y.; Kusoglu, A.; Karlsson, A.M.; Santare, M.H.; Cleghorn, S.; Johnson, W.B. Mechanical properties of a reinforced composite polymer electrolyte membrane and its simulated performance in PEM fuel cells. J. Power Sources 2008, 175, 817–825. [Google Scholar] [CrossRef] [Green Version]
- Kusoglu, A.; Savagatrup, S.; Clark, K.T.; Weber, A.Z. Role of mechanical factors in controlling the structure-function relationship of PFSA ionomers. Macromolecules 2012, 45, 7467–7476. [Google Scholar] [CrossRef]






| Annealing Temperature | Young’s Modulus (MPa) | Standard Deviation (%) |
|---|---|---|
| No annealed | 372 | 26.94 |
| 100 °C | 586 | 23.95 |
| 150 °C | 734 | 16.04 |
| 200 °C | 743 | 15.83 |
| 250 °C | 756 | 22.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pham, T.A.; Koo, S.; Park, H.; Luong, Q.T.; Kwon, O.J.; Jang, S.; Kim, S.M.; Kim, K. Investigation on the Microscopic/Macroscopic Mechanical Properties of a Thermally Annealed Nafion® Membrane. Polymers 2021, 13, 4018. https://doi.org/10.3390/polym13224018
Pham TA, Koo S, Park H, Luong QT, Kwon OJ, Jang S, Kim SM, Kim K. Investigation on the Microscopic/Macroscopic Mechanical Properties of a Thermally Annealed Nafion® Membrane. Polymers. 2021; 13(22):4018. https://doi.org/10.3390/polym13224018
Chicago/Turabian StylePham, Tuyet Anh, Seunghoe Koo, Hyunseok Park, Quang Thien Luong, Oh Joong Kwon, Segeun Jang, Sang Moon Kim, and Kyeongtae Kim. 2021. "Investigation on the Microscopic/Macroscopic Mechanical Properties of a Thermally Annealed Nafion® Membrane" Polymers 13, no. 22: 4018. https://doi.org/10.3390/polym13224018