Methylene Blue-Based Nano and Microparticles: Fabrication and Applications in Photodynamic Therapy
Abstract
:1. Introduction
2. Fabrication of Methylene Blue Nano and Microparticles
2.1. Methylene Blue in Polymeric Nano and Microparticles
2.2. Methylene Blue in Inorganic Nano and Microparticles
2.3. Methylene Blue in Inorganic Polymeric Composites
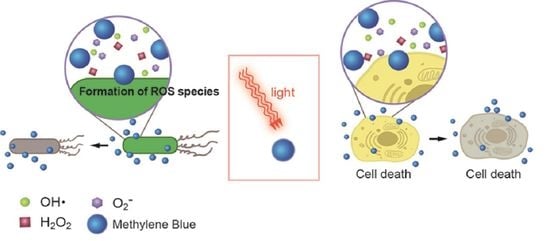
3. Methylene Blue in the Photodynamic Therapy
3.1. In-Vitro Observation of Methylene Blue (MB)-Mediated PDT in Cancer Treatment
3.2. In-Vitro and In-Vivo Applications of Methylene Blue (MB)-Mediated Antimicrobial Photodyanmic Therapy (aPDT)
4. Future Perspective on Methylene Blue Fabrications
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, G.; Nagbanshi, M.; Goldau, N.; Mendes Jorge, M.; Meissner, P.; Jahn, A.; Mockenhaupt, F.P.; Muller, O. Efficacy and safety of methylene blue in the treatment of malaria: A systematic review. BMC Med. 2018, 16, 59. [Google Scholar] [CrossRef]
- Vennerstrom, J.L.; Makler, M.T.; Angerhofer, C.K.; Williams, J.A. Antimalarial dyes revisited: Xanthenes, azines, oxazines, and thiazines. Antimicrob. Agents Chemother. 1995, 39, 2671–2677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oz, M.; Lorke, D.E.; Hasan, M.; Petroianu, G.A. Cellular and molecular actions of Methylene Blue in the nervous system. Med. Res. Rev. 2011, 31, 93–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ajithkumar, T.; Parkinson, C.; Shamshad, F.; Murray, P. Ifosfamide encephalopathy. Clin. Oncol. 2007, 19, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Ginimuge, P.R.; Jyothi, S.D. Methylene blue: Revisited. J. Anaesthesiol. Clin. Pharm. 2010, 26, 517–520. [Google Scholar]
- Juffermans, N.P.; Vervloet, M.G.; Daemen-Gubbels, C.R.G.; Binnekade, J.M.; Jong, M.d.; Groeneveld, A.B.J. A dose-finding study of methylene blue to inhibit nitric oxide actions in the hemodynamics of human septic shock. Nitric Oxide 2010, 22, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Galili, Y.; Kluger, Y.; Mianski, Z.; Iaina, A.; Wollman, Y.; Marmur, S.; Soffer, D.; Chernikovsky, T.; Klausner, J.P.; Robau, M.Y. Methylene blue—A promising treatment modality in sepsis induced by bowel perforation. Eur. Surg. Res. Eur. Chir. Forschung. Rech. Chir. Eur. 1997, 29, 390–395. [Google Scholar] [CrossRef]
- Park, B.-K.; Shim, T.-S.; Lim, C.-M.; Lee, S.-D.; Kim, W.-S.; Kim, D.-S.; Kim, W.-D.; Koh, Y. The effects of methylene blue on hemodynamic parameters and cytokine levels in refractory septic shock. Korean J. Intern. Med. 2005, 20, 123–128. [Google Scholar] [CrossRef]
- Atamna, H.; Kumar, R. Protective Role of Methylene Blue in Alzheimer’s Disease via Mitochondria and Cytochrome c Oxidase. J. Alzheimer’s Dis. 2010, 20, S439–S452. [Google Scholar] [CrossRef] [Green Version]
- Atamna, H.; Nguyen, A.; Schultz, C.; Boyle, K.; Newberry, J.; Kato, H.; Ames, B.N. Methylene blue delays cellular senescence and enhances key mitochondrial biochemical pathways. FASEB J. 2008, 22, 703–712. [Google Scholar] [CrossRef] [Green Version]
- Oz, M.; Lorke, D.E.; Petroianu, G.A. Methylene blue and Alzheimer’s disease. Biochem. Pharmacol. 2009, 78, 927–932. [Google Scholar] [CrossRef] [PubMed]
- Salman, A.E.; Salman, M.A.; Saricaoglu, F.; Akinci, S.B.; Aypar, Ü. Pain on injection of propofol: A comparison of methylene blue and lidocaine. J. Clin. Anesth. 2011, 23, 270–274. [Google Scholar] [CrossRef]
- Bojadzic, D.; Alcazar, O.; Buchwald, P. Methylene Blue Inhibits the SARS-CoV-2 Spike—ACE2 Protein-Protein Interaction—A Mechanism that can Contribute to its Antiviral Activity Against COVID-19. Front. Pharmacol. 2021, 11, 2255. [Google Scholar] [CrossRef] [PubMed]
- Clifton, J.I.; Leikin, J.B. Methylene Blue. Am. J. Ther. 2003, 10, 289–291. [Google Scholar] [CrossRef]
- Peter, C.; Hongwan, D.; Küpfer, A.; Lauterburg, B.H. Pharmacokinetics and organ distribution of intravenous and oral methylene blue. Eur. J. Clin. Pharmacol. 2000, 56, 247–250. [Google Scholar] [CrossRef]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef]
- Acedo, P.; Stockert, J.C.; Cañete, M.; Villanueva, A. Two combined photosensitizers: A goal for more effective photodynamic therapy of cancer. Cell Death Dis. 2014, 5, e1122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henderson, B.W.; Dougherty, T.J. How does photodynamic therapy work? Photochem. Photobiol. 1992, 55, 145–157. [Google Scholar] [CrossRef]
- Dougherty, T.J. Photodynamic therapy—New approaches. Semin. Surg. Oncol. 1989, 5, 6–16. [Google Scholar] [CrossRef]
- Dougherty, T.J.; Gomer, C.J.; Henderson, B.W.; Jori, G.; Kessel, D.; Korbelik, M.; Moan, J.; Peng, Q. Photodynamic Therapy. JNCI J. Natl. Cancer Inst. 1998, 90, 889–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dolmans, D.E.J.G.J.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef]
- Plaetzer, K.; Krammer, B.; Berlanda, J.; Berr, F.; Kiesslich, T. Photophysics and photochemistry of photodynamic therapy: Fundamental aspects. Lasers Med. Sci. 2009, 24, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Boltes Cecatto, R.; Siqueira de Magalhães, L.; Fernanda Setúbal Destro Rodrigues, M.; Pavani, C.; Lino-Dos-Santos-Franco, A.; Teixeira Gomes, M.; Fátima Teixeira Silva, D. Methylene blue mediated antimicrobial photodynamic therapy in clinical human studies: The state of the art. Photodiagn. Photodyn. Ther. 2020, 31, 101828. [Google Scholar] [CrossRef] [PubMed]
- Sibata, C.H.; Colussi, V.C.; Oleinick, N.L.; Kinsella, T.J. Photodynamic therapy in oncology. Expert Opin. Pharmacother. 2001, 2, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Xu, H.; Meyers, A.D.; Musani, A.I.; Wang, L.; Tagg, R.; Barqawi, A.B.; Chen, Y.K. Photodynamic therapy for treatment of solid tumors–potential and technical challenges. Technol. Cancer Res. Treat. 2008, 7, 309–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foote, C.S. Definition of type I and type II photosensitized oxidation. Photochem. Photobiol. 1991, 54, 659. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.-O.; Ha, K.-S. Chapter four—New Insights into the Mechanisms for Photodynamic Therapy-Induced Cancer Cell Death. In International Review of Cell and Molecular Biology; Jeon, K.W., Ed.; Academic Press: Cambridge, MA, USA, 2012; Volume 295, pp. 139–174. [Google Scholar]
- Mroz, P.; Yaroslavsky, A.; Kharkwal, G.B.; Hamblin, M.R. Cell Death Pathways in Photodynamic Therapy of Cancer. Cancers 2011, 3, 2516–2539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghorbani, J.; Rahban, D.; Aghamiri, S.; Teymouri, A.; Bahador, A. Photosensitizers in antibacterial photodynamic therapy: An overview. Laser Ther. 2018, 27, 293–302. [Google Scholar] [CrossRef] [Green Version]
- Vivas, R.; Barbosa, A.A.T.; Dolabela, S.S.; Jain, S. Multidrug-Resistant Bacteria and Alternative Methods to Control Them: An Overview. Microb. Drug Resist. 2019, 25, 890–908. [Google Scholar] [CrossRef]
- Siddique, S.; Chow, J.C.L. Application of Nanomaterials in Biomedical Imaging and Cancer Therapy. Nanomaterials 2020, 10, 1700. [Google Scholar] [CrossRef]
- Zhao, B.; Yin, J.-J.; Bilski, P.J.; Chignell, C.F.; Roberts, J.E.; He, Y.-Y. Enhanced photodynamic efficacy towards melanoma cells by encapsulation of Pc4 in silica nanoparticles. Toxicol. Appl. Pharm. 2009, 241, 163–172. [Google Scholar] [CrossRef] [Green Version]
- Maliszewska, I.; Wanarska, E.; Thompson, A.C.; Samuel, I.D.W.; Matczyszyn, K. Biogenic Gold Nanoparticles Decrease Methylene Blue Photobleaching and Enhance Antimicrobial Photodynamic Therapy. Molecules 2021, 26, 623. [Google Scholar] [CrossRef] [PubMed]
- Necula, M.; Breydo, L.; Milton, S.; Kayed, R.; van der Veer, W.E.; Tone, P.; Glabe, C.G. Methylene blue inhibits amyloid Abeta oligomerization by promoting fibrillization. Biochemistry 2007, 46, 8850–8860. [Google Scholar] [CrossRef]
- Lee, B.I.; Suh, Y.S.; Chung, Y.J.; Yu, K.; Park, C.B. Shedding Light on Alzheimer’s β-Amyloidosis: Photosensitized Methylene Blue Inhibits Self-Assembly of β-Amyloid Peptides and Disintegrates Their Aggregates. Sci. Rep. 2017, 7, 7523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taniguchi, S.; Suzuki, N.; Masuda, M.; Hisanaga, S.-i.; Iwatsubo, T.; Goedert, M.; Hasegawa, M. Inhibition of Heparin-induced Tau Filament Formation by Phenothiazines, Polyphenols, and Porphyrins. J. Biol. Chem. 2005, 280, 7614–7623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wischik, C.M.; Edwards, P.C.; Lai, R.Y.; Roth, M.; Harrington, C.R. Selective inhibition of Alzheimer disease-like tau aggregation by phenothiazines. Proc. Natl. Acad. Sci. USA 1996, 93, 11213–11218. [Google Scholar] [CrossRef] [Green Version]
- Rolla, G.; Bucca, C.; Brussino, L. Methylene blue in the hepatopulmonary syndrome. N. Engl. J. Med. 1994, 331, 1098. [Google Scholar] [CrossRef]
- Schenk, P.; Madl, C.; Rezaie-Majd, S.; Lehr, S.; Müller, C. Methylene blue improves the hepatopulmonary syndrome. Ann. Intern. Med. 2000, 133, 701–706. [Google Scholar] [CrossRef]
- Jounieaux, V.; Leleu, O.; Mayeux, I. Cardiopulmonary effects of nitric oxide inhalation and methylene blue injection in hepatopulmonary syndrome. Intensive Care Med. 2001, 27, 1103–1104. [Google Scholar] [CrossRef]
- Pelgrims, J.; De Vos, F.; Van den Brande, J.; Schrijvers, D.; Prové, A.; Vermorken, J.B. Methylene blue in the treatment and prevention of ifosfamide-induced encephalopathy: Report of 12 cases and a review of the literature. Br. J. Cancer 2000, 82, 291–294. [Google Scholar] [CrossRef]
- Küpfer, A.; Aeschlimann, C.; Wermuth, B.; Cerny, T. Prophylaxis and reversal of ifosfamide encephalopathy with methylene-blue. Lancet 1994, 343, 763–764. [Google Scholar] [CrossRef]
- Patel, P.N. Methylene Blue for Management of Ifosfamide-Induced Encephalopathy. Ann. Pharmacother. 2006, 40, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Färber, P.M.; Arscott, L.D.; Williams, C.H.; Becker, K.; Schirmer, R.H. Recombinant Plasmodium falciparum glutathione reductase is inhibited by the antimalarial dye methylene blue. FEBS Lett. 1998, 422, 311–314. [Google Scholar] [CrossRef] [Green Version]
- Schirmer, R.H.; Coulibaly, B.; Stich, A.; Scheiwein, M.; Merkle, H.; Eubel, J.; Becker, K.; Becher, H.; Müller, O.; Zich, T.; et al. Methylene blue as an antimalarial agent. Redox Rep. Commun. Free Radic. Res. 2003, 8, 272–275. [Google Scholar] [CrossRef] [Green Version]
- Meissner, P.E.; Mandi, G.; Coulibaly, B.; Witte, S.; Tapsoba, T.; Mansmann, U.; Rengelshausen, J.; Schiek, W.; Jahn, A.; Walter-Sack, I.; et al. Methylene blue for malaria in Africa: Results from a dose-finding study in combination with chloroquine. Malar. J. 2006, 5, 84. [Google Scholar] [CrossRef] [Green Version]
- Umbreit, J. Methemoglobin—It’s not just blue: A concise review. Am. J. Hematol. 2007, 82, 134–144. [Google Scholar] [CrossRef]
- McDonagh, E.M.; Bautista, J.M.; Youngster, I.; Altman, R.B.; Klein, T.E. PharmGKB summary: Methylene blue pathway. Pharm. Genom. 2013, 23, 498–508. [Google Scholar] [CrossRef] [Green Version]
- Wright, R.O.; Lewander, W.J.; Woolf, A.D. Methemoglobinemia: Etiology, Pharmacology, and Clinical Management. Ann. Emerg. Med. 1999, 34, 646–656. [Google Scholar] [CrossRef]
- Liao, Y.P.; Hung, D.Z.; Yang, D.Y. Hemolytic anemia after methylene blue therapy for aniline-induced methemoglobinemia. Vet. Hum. Toxicol. 2002, 44, 19–21. [Google Scholar]
- Koo, Y.-E.L.; Fan, W.; Hah, H.; Xu, H.; Orringer, D.; Ross, B.; Rehemtulla, A.; Philbert, M.A.; Kopelman, R. Photonic explorers based on multifunctional nanoplatforms for biosensing and photodynamic therapy. Appl. Opt. 2007, 46, 1924–1930. [Google Scholar] [CrossRef]
- Tang, W.; Xu, H.; Kopelman, R.; Philbert, M.A. Photodynamic characterization and in vitro application of methylene blue-containing nanoparticle platforms. Photochem. Photobiol. 2005, 81, 242–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, W.; Xu, H.; Park, E.J.; Philbert, M.A.; Kopelman, R. Encapsulation of methylene blue in polyacrylamide nanoparticle platforms protects its photodynamic effectiveness. Biochem. Biophys. Res. Commun. 2008, 369, 579–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tawfik, A.A.; Alsharnoubi, J.; Morsy, M. Photodynamic antibacterial enhanced effect of methylene blue-gold nanoparticles conjugate on Staphylococcal aureus isolated from impetigo lesions in vitro study. Photodiagn. Photodyn. Ther. 2015, 12, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, M.S.C.; Gouvêa, A.L.; de Moura, L.D.; Paterno, L.G.; de Souza, P.E.N.; Bastos, A.P.; Damasceno, E.A.M.; Veiga-Souza, F.H.; de Azevedo, R.B.; Báo, S.N. Nanographene oxide-methylene blue as phototherapies platform for breast tumor ablation and metastasis prevention in a syngeneic orthotopic murine model. J. Nanobiotechnol. 2018, 16, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khanal, A.; Bui, M.P.; Seo, S.S. Microgel-encapsulated methylene blue for the treatment of breast cancer cells by photodynamic therapy. J. Breast Cancer 2014, 17, 18–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hah, H.J.; Kim, G.; Lee, Y.-E.K.; Orringer, D.A.; Sagher, O.; Philbert, M.A.; Kopelman, R. Methylene Blue-Conjugated Hydrogel Nanoparticles and Tumor-Cell Targeted Photodynamic Therapy. Macromol. Biosci. 2011, 11, 90–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, M.; Hah, H.J.; Kim, G.; Nie, G.; Lee, Y.E.; Kopelman, R. Methylene blue covalently loaded polyacrylamide nanoparticles for enhanced tumor-targeted photodynamic therapy. Photochem. Photobiol. Sci. 2011, 10, 832–841. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Shen, Y.; Xu, L.; Xiang, G.; Ni, Z. Highly efficient and rapid adsorption of methylene blue dye onto vinyl hybrid silica nano-cross-linked nanocomposite hydrogel. Colloids Surf. A Physicochem. Eng. Asp. 2021, 613, 126050. [Google Scholar] [CrossRef]
- Chavanpatil, M.D.; Khdair, A.; Patil, Y.; Handa, H.; Mao, G.; Panyam, J. Polymer-surfactant nanoparticles for sustained release of water-soluble drugs. J Pharm. Sci. 2007, 96, 3379–3389. [Google Scholar] [CrossRef]
- Wu, P.T.; Lin, C.L.; Lin, C.W.; Chang, N.C.; Tsai, W.B.; Yu, J. Methylene-Blue-Encapsulated Liposomes as Photodynamic Therapy Nano Agents for Breast Cancer Cells. Nanomaterials 2018, 9, 14. [Google Scholar] [CrossRef] [Green Version]
- Hah, H.J.; Kim, J.S.; Jeon, B.J.; Koo, S.M.; Lee, Y.E. Simple preparation of monodisperse hollow silica particles without using templates. Chem. Commun. 2003, 1712–1713. [Google Scholar] [CrossRef]
- Monte, F.D.; Ferrer, M.L.; Levy, D. Probing the chemical environment at the porous cage of ormosils through the fluorescence of oxazine 1. J. Mater. Chem. 2001, 11, 1745–1751. [Google Scholar] [CrossRef]
- Sahu, K.; Roy, D.; Mondal, S.K.; Halder, A.; Bhattacharyya, K. Study of Solvation Dynamics in an Ormosil: CTAB in a Sol−Gel Matrix. J. Phys. Chem. B 2004, 108, 11971–11975. [Google Scholar] [CrossRef]
- Laranjo, M.T.; Stefani, V.; Benvenutti, E.V.; Costa, T.M.H.; Ramminger, G.d.O.; Gallas, M.R. Synthesis of ORMOSIL silica/rhodamine 6G: Powders and compacts. J. Non-Cryst. Solids 2007, 353, 24–30. [Google Scholar] [CrossRef]
- Dash, S.; Mishra, S.; Patel, S.; Mishra, B.K. Organically modified silica: Synthesis and applications due to its surface interaction with organic molecules. Adv. Colloid Interface Sci. 2008, 140, 77–94. [Google Scholar] [CrossRef]
- Dreaden, E.C.; Austin, L.A.; Mackey, M.A.; El-Sayed, M.A. Size matters: Gold nanoparticles in targeted cancer drug delivery. Ther. Deliv. 2012, 3, 457–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, J.A.; Chow, J.C.L. Recent progress and applications of gold nanotechnology in medical biophysics using artificial intelligence and mathematical modeling. Nano Express 2021, 2, 022001. [Google Scholar] [CrossRef]
- Siddique, S.; Chow, J.C.L. Gold Nanoparticles for Drug Delivery and Cancer Therapy. Appl. Sci. 2020, 10, 3824. [Google Scholar] [CrossRef]
- Vines, J.B.; Yoon, J.-H.; Ryu, N.-E.; Lim, D.-J.; Park, H. Gold Nanoparticles for Photothermal Cancer Therapy. Front. Chem. 2019, 7, 167. [Google Scholar] [CrossRef] [Green Version]
- Kimling, J.; Maier, M.; Okenve, B.; Kotaidis, V.; Ballot, H.; Plech, A. Turkevich Method for Gold Nanoparticle Synthesis Revisited. J. Phys. Chem. B 2006, 110, 15700–15707. [Google Scholar] [CrossRef]
- Park, J.-W.; Shumaker-Parry, J.S. Structural Study of Citrate Layers on Gold Nanoparticles: Role of Intermolecular Interactions in Stabilizing Nanoparticles. J. Am. Chem. Soc. 2014, 136, 1907–1921. [Google Scholar] [CrossRef] [PubMed]
- Amendola, V.; Pilot, R.; Frasconi, M.; Maragò, O.M.; Iatì, M.A. Surface plasmon resonance in gold nanoparticles: A review. J. Phys. Condens. Matter 2017, 29, 203002. [Google Scholar] [CrossRef] [PubMed]
- Maliszewska, I.; Leśniewska, A.; Olesiak-Bańska, J.; Matczyszyn, K.; Samoć, M. Biogenic gold nanoparticles enhance methylene blue-induced phototoxic effect on Staphylococcus epidermidis. J. Nanopart. Res. 2014, 16, 2457. [Google Scholar] [CrossRef]
- Khan, S.; Alam, F.; Azam, A.; Khan, A.U. Gold nanoparticles enhance methylene blue-induced photodynamic therapy: A novel therapeutic approach to inhibit Candida albicans biofilm. Int. J. Nanomed. 2012, 7, 3245–3257. [Google Scholar] [CrossRef] [Green Version]
- Perni, S.; Piccirillo, C.; Pratten, J.; Prokopovich, P.; Chrzanowski, W.; Parkin, I.P.; Wilson, M. The antimicrobial properties of light-activated polymers containing methylene blue and gold nanoparticles. Biomaterials 2009, 30, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Noimark, S.; Dunnill, C.; Kay, C.; Perni, S.; Prokopovich, P.; Ismail, S.; Wilson, M.; Parkin, I. Incorporation of methylene blue and nanogold into polyvinyl chloride catheters; A new approach for light-activated disinfection of surfaces. J. Mater. Chem. 2012, 22, 15388–15396. [Google Scholar] [CrossRef]
- Perni, S.; Piccirillo, C.; Kafizas, A.; Uppal, M.; Pratten, J.; Wilson, M.; Parkin, I.P. Antibacterial Activity of Light-Activated Silicone Containing Methylene Blue and Gold Nanoparticles of Different Sizes. J. Clust. Sci. 2010, 21, 427–438. [Google Scholar] [CrossRef]
- Link, E.M.; Brown, I.; Carpenter, R.N.; Mitchell, J.S. Uptake and therapeutic effectiveness of 125I- and 211At-methylene blue for pigmented melanoma in an animal model system. Cancer Res. 1989, 49, 4332–4337. [Google Scholar]
- Gabrielli, D.; Belisle, E.; Severino, D.; Kowaltowski, A.J.; Baptista, M.S. Binding, aggregation and photochemical properties of methylene blue in mitochondrial suspensions. Photochem. Photobiol. 2004, 79, 227–232. [Google Scholar] [CrossRef]
- Chen, Y.; Zheng, W.; Li, Y.; Zhong, J.; Ji, J.; Shen, P. Apoptosis induced by methylene-blue-mediated photodynamic therapy in melanomas and the involvement of mitochondrial dysfunction revealed by proteomics. Cancer Sci. 2008, 99, 2019–2027. [Google Scholar] [CrossRef]
- Bellin, J.S.; Mohos, S.C.; Oster, G. Dye-sensitized photoinactivation of tumor cells in vitro. Cancer Res. 1961, 21, 1365–1371. [Google Scholar]
- Yu, J.; Hsu, C.-H.; Huang, C.-C.; Chang, P.-Y. Development of Therapeutic Au–Methylene Blue Nanoparticles for Targeted Photodynamic Therapy of Cervical Cancer Cells. ACS Appl. Mater. Interfaces 2015, 7, 432–441. [Google Scholar] [CrossRef]
- Kofler, B.; Romani, A.; Pritz, C.; Steinbichler, T.B.; Schartinger, V.H.; Riechelmann, H.; Dudas, J. Photodynamic Effect of Methylene Blue and Low Level Laser Radiation in Head and Neck Squamous Cell Carcinoma Cell Lines. Int. J. Mol. Sci. 2018, 19, 1107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, E.J.; Oak, C.-H.; Heo, J.; Kim, Y.-H. Methylene blue-mediated photodynamic therapy enhances apoptosis in lung cancer cells. Oncol. Rep. 2013, 30, 856–862. [Google Scholar] [CrossRef]
- Dos Santos, A.F.; Terra, L.F.; Wailemann, R.A.M.; Oliveira, T.C.; Gomes, V.d.M.; Mineiro, M.F.; Meotti, F.C.; Bruni-Cardoso, A.; Baptista, M.S.; Labriola, L. Methylene blue photodynamic therapy induces selective and massive cell death in human breast cancer cells. BMC Cancer 2017, 17, 194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsubara, T.; Kusuzaki, K.; Matsumine, A.; Satonaka, H.; Shintani, K.; Nakamura, T.; Uchida, A. Methylene blue in place of acridine orange as a photosensitizer in photodynamic therapy of osteosarcoma. In Vivo 2008, 22, 297–303. [Google Scholar]
- Guan, J.; Lai, X.; Wang, X.; Leung, A.W.; Zhang, H.; Xu, C. Photodynamic action of methylene blue in osteosarcoma cells in vitro. Photodiagn. Photodyn. Ther. 2014, 11, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Zeina, B.; Greenman, J.; Purcell, W.M.; Das, B. Killing of cutaneous microbial species by photodynamic therapy. Br. J. Derm. 2001, 144, 274–278. [Google Scholar] [CrossRef]
- Wainwright, M.; Phoenix, D.A.; Marland, J.; Wareing, D.R.A.; Bolton, F.J. A study of photobactericidal activity in the phenothiazinium series. FEMS Immunol. Med. Microbiol. 1997, 19, 75–80. [Google Scholar] [CrossRef]
- Ventola, C.L. The antibiotic resistance crisis: Part 1: Causes and threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Zolfaghari, P.S.; Packer, S.; Singer, M.; Nair, S.P.; Bennett, J.; Street, C.; Wilson, M. In vivo killing of Staphylococcus aureus using a light-activated antimicrobial agent. BMC Microbiol. 2009, 9, 27. [Google Scholar] [CrossRef] [Green Version]
- Pereira, A.H.C.; Pinto, J.G.; Freitas, M.A.A.; Fontana, L.C.; Pacheco Soares, C.; Ferreira-Strixino, J. Methylene blue internalization and photodynamic action against clinical and ATCC Pseudomonas aeruginosa and Staphyloccocus aureus strains. Photodiagn. Photodyn. Ther. 2018, 22, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Hu, Y.; Zhang, L.-P.; Liu, B.; Yang, Y.; Tang, T.; Tian, J.; Peng, K.; Liu, T. Lactic-co-glycolic acid-coated methylene blue nanoparticles with enhanced antibacterial activity for efficient wound healing. RSC Adv. 2020, 10, 12304–12307. [Google Scholar] [CrossRef]
- Uckay, I.; Gariani, K.; Pataky, Z.; Lipsky, B.A. Diabetic foot infections: State-of-the-art. Diabetes Obes. Metab. 2014, 16, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Loesche, W.J. Role of Streptococcus mutans in human dental decay. Microbiol. Rev. 1986, 50, 353–380. [Google Scholar] [CrossRef]
- Mendez, D.A.C.; Gutierrez, E.; Dionisio, E.J.; Oliveira, T.M.; Buzalaf, M.A.R.; Rios, D.; Machado, M.; Cruvinel, T. Effect of methylene blue-mediated antimicrobial photodynamic therapy on dentin caries microcosms. Lasers Med. Sci. 2018, 33, 479–487. [Google Scholar] [CrossRef]
- Leal, C.R.L.; Alvarenga, L.H.; Oliveira-Silva, T.; Kato, I.T.; Godoy-Miranda, B.; Bussadori, S.K.; Ribeiro, M.S.; Prates, R.A. Antimicrobial photodynamic therapy on Streptococcus mutans is altered by glucose in the presence of methylene blue and red LED. Photodiagn. Photodyn. Ther. 2017, 19, 1–4. [Google Scholar] [CrossRef]
- Huang, T.-C.; Chen, C.-J.; Ding, S.-J.; Chen, C.-C. Antimicrobial efficacy of methylene blue-mediated photodynamic therapy on titanium alloy surfaces in vitro. Photodiagn. Photodyn. Ther. 2019, 25, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front Pharm. 2015, 6, 286. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Li, X.; Zhao, C. Strategies to Obtain Encapsulation and Controlled Release of Small Hydrophilic Molecules. Front. Bioeng. Biotechnol. 2020, 8, 437. [Google Scholar] [CrossRef]
- Boccalini, G.; Conti, L.; Montis, C.; Bani, D.; Bencini, A.; Berti, D.; Giorgi, C.; Mengoni, A.; Valtancoli, B. Methylene blue-containing liposomes as new photodynamic anti-bacterial agents. J. Mater. Chem. B 2017, 5, 2788–2797. [Google Scholar] [CrossRef] [PubMed]
- Vrignaud, S.; Benoit, J.-P.; Saulnier, P. Strategies for the nanoencapsulation of hydrophilic molecules in polymer-based nanoparticles. Biomaterials 2011, 32, 8593–8604. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Indications | Descriptions | Ref. |
|---|---|---|
| Alzheimer’s disease | Positive effects are proposed through multiple neurological systems such as cholinergic, serotonergic and glutamatergic neurotransmitter systems MB reduces aggregated amyloid-β (Aβ) peptide while preventing tau aggregation A recommended dose of 3 × 60 mg/day for treatment | [11,34,35,36,37] |
| Hepatopulmonary Syndrome | Intravenous dose of MB (3 mg per kg) for treatment | [38,39,40] |
| Ifosfamide-induced encephalopahty | Intravenous dose of MB (6 × 50 mg/day) for treatment | [41,42,43] |
| Malaria | MB has the high antimalarial potency (IC50 = 4 nM) against Plasmodium falciparum MB inhibits P. falciparum glutathione reductase (PfGR) known as a drug target against Malaria MB is a partner drug for combination therapies | [2,44,45,46] |
| Methemoglobinemia | MB is an electron donor for the non-enzymatic reduction of methemoglobin | [47,48,49] 1 |
| Strains | Minimum Lethal Concentration (µM) | Type of Bacteria | |
|---|---|---|---|
| Dark | Light | ||
| Staphylococcus aureus | 2.5 | 1 | Gram-negative |
| Enterococcus faecalis | 500 | 100 | |
| Bacillus cereus | 1000 | 1000 | |
| Escherichia coli | 100 | 100 | Gram-positive |
| Pseudomonas aeruginosa | 500 | 125 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, D.-J. Methylene Blue-Based Nano and Microparticles: Fabrication and Applications in Photodynamic Therapy. Polymers 2021, 13, 3955. https://doi.org/10.3390/polym13223955
Lim D-J. Methylene Blue-Based Nano and Microparticles: Fabrication and Applications in Photodynamic Therapy. Polymers. 2021; 13(22):3955. https://doi.org/10.3390/polym13223955
Chicago/Turabian StyleLim, Dong-Jin. 2021. "Methylene Blue-Based Nano and Microparticles: Fabrication and Applications in Photodynamic Therapy" Polymers 13, no. 22: 3955. https://doi.org/10.3390/polym13223955