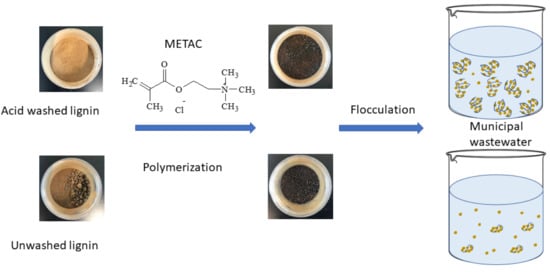
Cationic Lignin Polymers as Flocculant for Municipal Wastewater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthetic Wastewater
2.3. Lignin METAC Polymer Synthesis
2.4. Charge Density, Solubility, and Elemental Analyses
2.5. Hydroxyl Group Analysis
2.6. Molecular Weight Analysis
2.7. Thermal Gravimetric Analysis (TGA)
2.8. Flocculation and Coagulation of Wastewater
2.9. Chemical Oxygen Demand (COD) and Total Organic Carbon (TOC) Analysis
2.10. Total Ammonia and Phosphorous Analysis
2.11. Focused Beam Reflectance Measurement (FBRM)
3. Results and Discussion
3.1. Characteristics of Unmodified Lignin
3.2. Characteristics of Lignin–METAC
3.3. Thermal Properties
3.4. Flocculation of Wastewater by Unmodified Lignin
3.5. Flocculation of Wastewater by Lignin–METAC Polymer
3.6. Floc Formation
3.7. Dual Coagulation–Flocculation System
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Gao, B.; Yue, Q.; Wang, Y. Effect of viscosity, basicity and organic content of composite flocculant on the decolorization performance and mechanism for reactive dyeing wastewater. J. Environ. Sci. 2011, 23, 1626–1633. [Google Scholar] [CrossRef]
- Lee, C.S.; Chong, M.F.; Robinson, J.; Binner, E. A review on development and application of plant-based bioflocculants and grafted bioflocculants. Ind. Eng. Chem. Res. 2014, 53, 18357–18369. [Google Scholar] [CrossRef] [Green Version]
- Brostow, W.; Lobland, H.; Pal, S.; Singh, R. Polymeric flocculants for wastewater and industrial effluent treatment. J. Mater. Educ. 2009, 31, 157–166. [Google Scholar]
- Lee, C.S.; Robinson, J.; Chong, M.F. A review on application of flocculants in wastewater treatment. Process. Saf. Environ. Prot. 2014, 92, 489–508. [Google Scholar] [CrossRef]
- Aguilar, M.I.; Saez, J.; Llorens, M.; Soler, A.; Ortuno, J.F. Nutrient removal and sludge production in the coagulation–flocculation process. Water Res. 2002, 36, 2910–2919. [Google Scholar] [CrossRef]
- Nir, T.; Arkhangelsky, E.; Levitsky, I.; Gitis, V. Removal of phosphorus from secondary effluents by coagulation and ultrafiltration. Desalination Water Treat. 2009, 8, 24–30. [Google Scholar] [CrossRef]
- Dlamini, N.G.; Basson, A.K.; Pullabhotla, R.V. Wastewater treatment by a polymeric bioflocculant and iron nanoparticles synthesized from a bioflocculant. Polymers 2020, 12, 1618. [Google Scholar] [CrossRef]
- Fang, R.; Cheng, X.; Xu, X. Synthesis of lignin-base cationic flocculant and its application in removing anionic azo-dyes from simulated wastewater. Bioresour. Technol. 2010, 101, 7323–7329. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Hao, C.; Dai, X.; Zhou, Z.; Si, N. Ultrasonic-assisted synthesis of aminated lignin by a Mannich reaction and its decolorizing properties for anionic azo-dyes. RSC Adv. 2014, 4, 28156–28164. [Google Scholar] [CrossRef]
- Kuutti, L.; Haavisto, S.; Hyvarinen, S.; Mikkonen, H.; Koski, R.; Peltonen, S.; Suortti, T.; Kyllönen, H. Properties and flocculation efficiency of cationized biopolymers and their applicability in papermaking and in conditioning of pulp and paper sludge. BioResources 2011, 6, 2836–2850. [Google Scholar]
- Harmita, H.; Karthikeyan, K.G.; Pan, X. Copper and cadmium sorption onto kraft and organosolv lignins. Bioresour. Technol. 2009, 100, 6183–6191. [Google Scholar] [CrossRef]
- Lü, Q.F.; Huang, Z.K.; Liu, B.; Cheng, X. Preparation and heavy metal ions biosorption of graft copolymers from enzymatic hydrolysis lignin and amino acids. Bioresour. Technol. 2012, 104, 111–118. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Meng, X.; Pu, Y.; Ragauskas, A.J. Recent advances in the application of functionalized lignin in value-added polymeric materials. Polymers 2020, 12, 2277. [Google Scholar] [CrossRef]
- Albadarin, A.B.; Al-Muhtaseb, A.H.; Al-laqtah, A.A.; Al-Muhtaseb, H.; Al-Laqtah, N.A.; Walker, G.M.; Allen, S.J.; Ahmad, M.N.M. Biosorption of toxic chromium from aqueous phase by lignin: Mechanism, effect of other metal ions and salts. Chem. Eng. J. 2011, 169, 20–30. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, D.; Bei, Y.; Ren, S.; Fang, G. Flocculation performance of trimethyl quaternary ammonium salt of lignin-sodium alginate polyampholyte. BioResources 2013, 8, 3544–3555. [Google Scholar] [CrossRef] [Green Version]
- Ozgun, H.; Dereli, R.K.; Ersahin, M.E.; Kinaci, C.; Spanjers, H.; van Lier, J.B. A review of anaerobic membrane bioreactors for municipal wastewater treatment: Integration options, limitations and expectations. Sep. Purif. Technol. 2013, 118, 89–104. [Google Scholar] [CrossRef]
- Du, X.; Li, J.; Lindström, M.E. Modification of industrial softwood kraft lignin using Mannich reaction with and without phenolation pretreatment. Ind. Crop. Prod. 2014, 52, 729–735. [Google Scholar] [CrossRef]
- Laurichesse, S.; Avérous, L. Chemical modification of lignins: Towards biobased polymers. Prog. Polym. Sci. 2014, 39, 1266–1290. [Google Scholar] [CrossRef]
- Wang, S.; Kong, F.; Gao, W.; Fatehi, P. Novel process for generating cationic lignin based flocculant. Ind. Eng. Chem. Res. 2018, 57, 6595–6608. [Google Scholar] [CrossRef]
- Wang, S.; Kong, F.; Fatehi, P.; Hou, Q. Cationic High Molecular Weight Lignin Polymer: A Flocculant for the Removal of Anionic Azo-Dyes from Simulated Wastewater. Molecules 2018, 23, 2005. [Google Scholar] [CrossRef] [Green Version]
- Sheldon, R.A. Green solvents for sustainable organic synthesis: State of the art. Green Chem. 2005, 7, 267–278. [Google Scholar] [CrossRef]
- Wang, J.P.; Yuan, S.J.; Wang, Y.; Yu, H.Q. Synthesis, characterization and application of a novel starch-based flocculant with high flocculation and dewatering properties. Water Res. 2013, 47, 2643–2648. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Panda, A.B.; Pal, S. Synthesis and characterization of a novel polymeric hydrogel based on hydroxypropyl methyl cellulose grafted with polyacrylamide. Cellulose 2012, 19, 933–945. [Google Scholar] [CrossRef]
- Giummarella, N.; Lindgren, C.; Lindström, M.; Henriksson, G. Lignin prepared by ultrafiltration of black liquor: Investigation of solubility, viscosity, and ash content. BioResources 2016, 11, 3494–3510. [Google Scholar] [CrossRef] [Green Version]
- Sahoo, S.; Seydibeyo Glu, M.; Mohanty, A.K.; Misra, M. Characterization of industrial lignins for their utilization in future value added applications. Biomass Bioenergy 2011, 35, 4230–4237. [Google Scholar] [CrossRef]
- Zhu, W.; Theliander, H. Precipitation of lignin from softwood black liquor: An investigation of the equilibrium and molecular properties of lignin. BioResources 2015, 10, 1696. [Google Scholar] [CrossRef] [Green Version]
- He, L.; Liu, Q.; Song, Y.; Deng, Y. Effects of metal chlorides on the solubility of lignin in the black liquor of prehydrolysis kraft pulping. BioResources 2014, 9, 4636. [Google Scholar] [CrossRef] [Green Version]
- Nopens, I.; Capalozza, C.; Vanrolleghem, P.A. Stability Analysis of a Synthetic Municipal Wastewater; Department of Applied Mathematics Biometrics and Process Control, University of Gent: Ghent, Belgium, 2001. [Google Scholar]
- Couch, R.L.; Price, J.T.; Fatehi, P. Production of flocculant from thermomechanical pulping lignin via nitric acid treatment. ACS Sustain. Chem. Eng. 2016, 4, 1954–1962. [Google Scholar] [CrossRef]
- Hopa, D.Y.; Fatehi, P. Using sulfobutylated and sulfomethylated lignin as dispersant for kaolin suspension. Polymers 2020, 12, 2046. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, Y.; Iwatsuki, A.; Yasuda, S. Application of cationic polymer prepared from sulfuric acid lignin as a retention aid for usual rosin sizes to neutral papermaking. J. Wood Sci. 2004, 50, 540–544. [Google Scholar] [CrossRef] [Green Version]
- Crestini, C.; Argyropoulos, D.S. Structural Analysis of Wheat Straw Lignin by Quantitative 31P and 2D NMR Spectroscopy. The Occurrence of Ester Bonds and α-O-4 Substructures. J. Agric. Food Chem. 1997, 45, 1212–1219. [Google Scholar] [CrossRef]
- Pu, Y.; Cao, S.; Ragauskas, A.J. Application of quantitative 31P NMR in biomass lignin and biofuel precursors characterization. Energy Environ. Sci. 2011, 4, 3154–3166. [Google Scholar] [CrossRef]
- Chen, X.; Si, C.; Fatehi, P. Pretreatment and in situ fly ash systems for improving the performance of sequencing batch reactor in treating thermomechanical pulping effluent. ACS Sustain. Chem. Eng. 2017, 5, 6932–6939. [Google Scholar] [CrossRef]
- Yu, Z.Q.; Chow, P.S.; Tan, R.B.H. Interpretation of focused beam reflectance measurement (FBRM) data via simulated crystallization. Org. Process. Res. Dev. 2008, 12, 646–654. [Google Scholar] [CrossRef]
- Jiang, X.; Savithri, D.; Du, X.; Pawar, S.; Jameel, H.; Chang, H.-M.; Zhou, X. Fractionation and characterization of kraft lignin by sequential precipitation with various organic solvents. ACS Sustain. Chem. Eng. 2017, 5, 835–842. [Google Scholar] [CrossRef]
- Chakar, F.S.; Ragauskas, A.J. Review of current and future softwood kraft lignin process chemistry. Ind. Crop. Prod. 2004, 20, 131–141. [Google Scholar] [CrossRef]
- Froass, P.M.; Ragauskas, A.J.; Jiang, J.-E. Chemical structure of residual lignin from kraft pulp. J. Wood Chem. Technol. 1996, 16, 347–365. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, K.; Li, J.; Yang, G.; Liu, S.; Xu, J. The solubility of lignin from bagasse in a 1,4-butanediol/water system. BioResources 2011, 6, 3034. [Google Scholar]
- Duval, A.; Lawoko, M. A review on lignin-based polymeric, micro-and nano-structured materials. React. Funct. Polym. 2014, 85, 78–96. [Google Scholar] [CrossRef]
- El Mansouri, N.-E.; Salvadó, J. Structural characterization of technical lignins for the production of adhesives: Application to lignosulfonate, kraft, soda-anthraquinone, organosolv and ethanol process lignins. Ind. Crop. Prod. 2006, 24, 8–16. [Google Scholar] [CrossRef]
- Jääskeläinen, A.S.; Sun, Y.; Argyropoulos, D.S.; Tamminen, T.; Hortling, B. The effect of isolation method on the chemical structure of residual lignin. Wood Sci. Technol. 2003, 37, 91–102. [Google Scholar] [CrossRef]
- Sivasankarapillai, G.; McDonald, A.G. Synthesis and properties of lignin-highly branched poly (ester-amine) polymeric systems. Biomass Bioenergy 2011, 35, 919–931. [Google Scholar] [CrossRef]
- Kong, F.; Wang, S.; Price, J.T.; Konduri, M.K.R.; Fatehi, P. Water soluble kraft lignin–acrylic acid copolymer: Synthesis and characterization. Green Chem. 2015, 17, 4355–4366. [Google Scholar] [CrossRef]
- Woodworth, B.E.; Metzner, Z.; Matyjaszewski, K. Copper triflate as a catalyst in atom transfer radical polymerization of styrene and methyl acrylate. Macromolecules 1998, 31, 7999–8004. [Google Scholar] [CrossRef]
- Singht, B.B.; Ormerod, M.G. The effect of metal ions on free radical formation and reactions in irradiated proteins. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1966, 10, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Thielemans, W.; Can, E.; Morye, S.S.; Wool, R.P. Novel applications of lignin in composite materials. J. Appl. Polym. Sci. 2002, 83, 323–331. [Google Scholar] [CrossRef]
- Dorez, G.; Ferry, L.; Sonnier, R.; Taguet, A.; Lopez-Cuesta, J.-M. Effect of cellulose, hemicellulose and lignin contents on pyrolysis and combustion of natural fibers. J. Anal. Appl. Pyrolysis 2014, 107, 323–331. [Google Scholar] [CrossRef]
- Goel, N.K.; Rao, M.S.; Kumar, V.; Bhardwaj, Y.K.; Chaudhari, C.V.; Dubey, K.A.; Sabharwal, S. Synthesis of antibacterial cotton fabric by radiation-induced grafting of [2-(Methacryloyloxy)ethyl] trimethylammonium chloride (MAETC) onto cotton. Radiat. Phys. Chem. 2009, 78, 399–406. [Google Scholar] [CrossRef]
- Gomes, M.D.L.I.; Osório, E.; Vilela, A.C.F. Thermal analysis evaluation of the reactivity of coal mixtures for injection in the blast furnace. Mater. Res. 2006, 9, 91–95. [Google Scholar] [CrossRef]
- Nasser, M.S.; James, A.E. The effect of polyacrylamide charge density and molecular weight on the flocculation and sedimentation behaviour of kaolinite suspensions. Sep. Purif. Technol. 2006, 52, 241–252. [Google Scholar] [CrossRef]
- Chon, K.; KyongShon, H.; Cho, J. Membrane bioreactor and nanofiltration hybrid system for reclamation of municipal wastewater: Removal of nutrients, organic matter and micropollutants. Bioresour. Technol. 2012, 122, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Suopajärvi, T.; Liimatainen, H.; Hormi, O.; Niinimäki, J. Coagulation–flocculation treatment of municipal wastewater based on anionized nanocelluloses. Chem. Eng. J. 2013, 231, 59–67. [Google Scholar] [CrossRef]
- Blanco, A.; Fuente, E.; Negro, C.; Tijero, J. Flocculation monitoring: Focused beam reflectance measurement as a measurement tool. Can. J. Chem. Eng. 2002, 80, 1–7. [Google Scholar] [CrossRef]
- Owen, A.; Fawell, P.; Swift, J.; Farrow, J.; Parker, A. The impact of polyacrylamide flocculant solution age on flocculation performance. Int. J. Miner. Process. 2002, 67, 123–144. [Google Scholar] [CrossRef]
- Biggs, S.; Habgood, M.; Jameson, G.J.; Yan, Y.D. Aggregate structures formed via a bridging flocculation mechanism. Chem. Eng. J. 2000, 80, 13–22. [Google Scholar] [CrossRef]
- Zhou, Y.; Franks, G.V. Flocculation mechanism induced by cationic polymers investigated by light scattering. Langmuir 2006, 22, 6775–6786. [Google Scholar] [CrossRef] [PubMed]
- Saleh, M.Y.; El Enany, G.; Elzahar, M.H.; Elshikhipy, M.Z. Industrial Wastewater Treatment Using High Rate Activated Sludge and Alum Additive. Int. J. Environ. Sci. Dev. 2014, 5, 551–556. [Google Scholar] [CrossRef] [Green Version]
- Guida, M.; Mattei, M.; Della Rocca, C.; Melluso, G.; Meriç, S. Optimization of alum-coagulation/flocculation for COD and TSS removal from five municipal wastewater. Desalination 2007, 211, 113–127. [Google Scholar] [CrossRef]
- Bao, H.; Liu, Y.; Jia, H. A study of irradiation in the treatment of wastewater. Radiat. Phys. Chem. 2002, 63, 633–636. [Google Scholar] [CrossRef]
- Hameed, Y.T.; Idris, A.; Hussain, S.A.; Abdullah, N. A tannin-based agent for coagulation and flocculation of municipal wastewater: Chemical composition, performance assessment compared to Polyaluminum chloride, and application in a pilot plant. J. Environ. Manag. 2016, 184, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Beltrán-Heredia, J.; Sánchez-Martín, J. Municipal wastewater treatment by modified tannin flocculant agent. Desalination 2009, 249, 353–358. [Google Scholar] [CrossRef]




| Charge Density (meq/g) | Solubility (wt %) | Organic Elements (wt %) | Inorganic Elements (wt %) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | H | N | S | O | Al | Ca | Fe | Na | K | Total Inorganics | |||
| AWL1 | NA * | 7.2 | 65.2 | 5.2 | 0.0 | 2.4 | 26.9 | <0.01 | 0.01 | <0.01 | 0.31 | 0.03 | 2.05 |
| AWL2 | NA | 7.5 | 64.0 | 5.1 | 0.0 | 2.2 | 27.0 | <0.01 | <0.01 | <0.01 | 0.18 | 0.02 | 1.74 |
| UWL1 | −1.77 | 94 | 49.5 | 4.3 | 0.0 | 1.4 | 32.9 | 0.01 | 0.08 | <0.01 | 7.51 | 0.98 | 10.40 |
| UWL2 | −1.78 | 92 | 54.1 | 4.5 | 0.0 | 1.7 | 31.6 | 0.01 | 0.02 | 0.01 | 5.37 | 0.63 | 7.61 |
| Non-Phenolic Hydroxyl Groups | Phenolic Hydroxyl Groups | ||||||
|---|---|---|---|---|---|---|---|
| Aliphatic | Carboxylic | Coniferyl | Sinapyl | P-Coumaryl | C5 Substituted | Total | |
| AWL1 | 1.13 | 0.26 | 1.09 | 0.46 | 0.09 | 0.96 | 2.60 |
| AWL2 | 1.36 | 0.17 | 1.36 | 0.65 | 0.05 | 1.30 | 3.36 |
| Mw (kg/mol) | Solubility (wt %) | Charge Density (meq/g) | |
|---|---|---|---|
| AM1 | 210 ± 90 | 94 | 2.3 |
| AM2 | 140 ± 50 | 96 | 3.2 |
| UM1 | 76 ± 9 | 90 | 2.6 |
| UM2 | 70 ± 20 | 89 | 3.3 |
| Peak Temperature (°C) | DTGmax (%/°C) | WR (%) | R (%/kg·s) | |||
|---|---|---|---|---|---|---|
| TP1 | TP2 | TP3 | ||||
| Unmodified Lignin | ||||||
| AWL1 | 46.4 | 437 | - | 0.173 | 36.2 | 27,900 |
| AWL2 | 41.7 | 414 | - | 0.181 | 30.8 | 29,200 |
| UWL1 | 50.3 | 330 | - | 0.181 | 58.8 | 30,100 |
| UWL2 | 56.6 | 331 | - | 0.184 | 58.0 | 29,200 |
| Lignin–METAC polymers | ||||||
| AM1 | 58.4 | 278 | 437 | 0.410 | 11.4 | 66,200 |
| AM2 | 67.2 | 287 | 441 | 0.436 | 23.5 | 70,300 |
| UM1 | 58.4 | 281 | 449 | 0.456 | 18.2 | 76,000 |
| UM2 | 58.5 | 277 | 444 | 0.506 | 15.0 | 80,200 |
| Optimum Flocculant Dosage (mg/L) | COD Removal, % | TOC Removal, % | ||
|---|---|---|---|---|
| Theoretical 1 | Experimental | |||
| AM1 | 75.4 | 67 | 23 ± 5 | 51 ± 3 |
| AM2 | 54.1 | 47 | 19 ± 5 | 59 ± 3 |
| UM1 | 67.0 | 51 | 17 ± 5 | 60 ± 3 |
| UM2 | 51.3 | 48 | 19 ± 5 | 57 ± 3 |
| Dosage of Lignin, mg/L | Dosage of Alum, mg/L | Total Inorganic Content, ±0.5 mg/L | NH3, ±1 mg·L | Phosphorous, ±0.2 mg/L | COD, ±5 mg/L | |
|---|---|---|---|---|---|---|
| Wastewater | 0 | 0 | 62.1 | 36 | 8.8 | 257 |
| AWL1 | 65 | 0 | 60.2 | 33 | 6.0 | 275 |
| AM1 | 65 | 0 | 61.2 | 44 | 8.5 | 209 |
| Alum + AM1 | 65 | 1 | 59.9 | 44 | 5.1 | 144 |
| Alum | 0 | 200 | 95.0 | 24 | 2.2 | 145 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moore, C.; Gao, W.; Fatehi, P. Cationic Lignin Polymers as Flocculant for Municipal Wastewater. Polymers 2021, 13, 3871. https://doi.org/10.3390/polym13223871
Moore C, Gao W, Fatehi P. Cationic Lignin Polymers as Flocculant for Municipal Wastewater. Polymers. 2021; 13(22):3871. https://doi.org/10.3390/polym13223871
Chicago/Turabian StyleMoore, Courtney, Weijue Gao, and Pedram Fatehi. 2021. "Cationic Lignin Polymers as Flocculant for Municipal Wastewater" Polymers 13, no. 22: 3871. https://doi.org/10.3390/polym13223871