Operational Stability, Regenerability, and Thermodynamics Studies on Biogenic Silica/Magnetite/Graphene Oxide Nanocomposite-Activated Candida rugosa Lipase
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
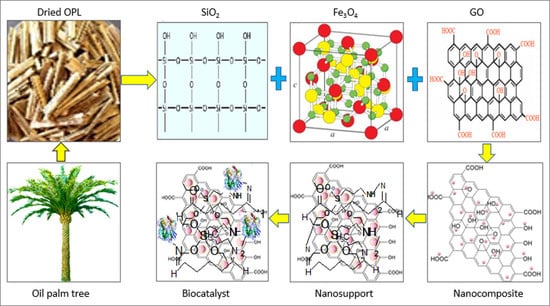
2.2. Preparation of Support Systems
2.2.1. Preparation of Biogenic Silica, Graphene Oxide, and Magnetic Graphene Oxide
2.2.2. Preparation and Modification of the SiO2/Fe3O4/GO Nanocomposite
2.3. Esterification Synthesis of EV Catalyzed by CRL/SiO2/Fe3O4/GO
2.4. Characterization of Support Matrix and Biocatalyst
2.4.1. Chemical Composition, Oxidation States, and Microcrystalline Structure Analysis
2.4.2. Textural Properties and Pore Structure Analysis
2.4.3. Surface Morphological and Microstructural Analysis
2.4.4. Magnetic Behavior Analysis
2.4.5. Chemical Composition and Functional Group Analysis
2.5. Effect of Reaction Time on Esterification of Ethanol and Valeric Acid
2.6. Operational Stability Studies
2.6.1. Thermal Stability
2.6.2. Half-Life (T50)
2.6.3. Storage Stability
2.6.4. Regeneration Study
2.7. Thermodynamic Study-Effect of Temperature on Free CRL and CRL/SiO2/Fe3O4/GO
2.8. Characterization of Esterification Product
2.9. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Supports and Biocatalyst
3.1.1. Chemical Composition and Microcrystalline Structure Analysis
3.1.2. Textural Properties and Pore Structure Analysis
3.1.3. Surface Morphological and Microstructural Analysis
3.1.4. Magnetic Behavior Analysis
3.1.5. Chemical Composition and Functional Group Analysis
3.2. Effect of Reaction Time on EV Synthesis
3.3. Operational Stability Studies
3.3.1. Thermal Stability and Half-Life
3.3.2. Short-Term Storage Stability
3.3.3. Regeneration Study
3.4. Thermodynamic Study
3.4.1. Kinetic Rate Constant and Activation Energy
3.4.2. Deactivation Rate Constant and Deactivation Energy
3.4.3. Thermodynamics Parameters
3.5. Product Identification and Structural Elucidation of Ethyl Valerate
Gas Chromatography and Proton Nuclear Magnetic Resonance Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jacob, A.G.; Wahab, R.A.; Mahat, N.A. Ternary biogenic silica/magnetite/graphene oxide composite for the hyperactivation of Candida rugosa lipase in the esterification production of ethyl valerate. Enzym. Microb. Technol. 2021, 148, 109807. [Google Scholar] [CrossRef]
- Xie, W.; Wang, H. Immobilized polymeric sulfonated ionic liquid on core-shell structured Fe3O4/SiO2 composites: A magnetically recyclable catalyst for simultaneous transesterification and esterification of low-cost oils to biodiesel. Renew. Energy 2020, 145, 1709–1719. [Google Scholar] [CrossRef]
- Onoja, E.; Chandren, S.; Razak, F.I.; Wahab, R.A. Extraction of nanosilica from oil palm leaves and its application as support for lipase immobilization. J. Biotechnol. 2018, 283, 81–96. [Google Scholar] [CrossRef] [PubMed]
- Onoja, E.; Wahab, R.A. Effect of glutaraldehyde concentration on catalytic efficacy of Candida rugosa lipase immobilized onto silica from oil palm leaves. Indones. J. Chem. 2019, 19, 1043–1054. [Google Scholar] [CrossRef] [Green Version]
- Wong, W.K.; Wahab, R.A.; Onoja, E. Chemically modified nanoparticles from oil palm ash silica-coated magnetite as support for Candida rugosa lipase-catalysed hydrolysis: Kinetic and thermodynamic studies. Chem. Pap. 2020, 4, 1253–1265. [Google Scholar] [CrossRef]
- Onoja, E.; Wahab, R.A. Robust magnetized oil palm leaves ash nanosilica composite as lipase support: Immobilization protocol and efficacy study. Appl. Biochem. Biotechnol. 2020, 192, 585–599. [Google Scholar] [CrossRef]
- Gustafsson, H.; Johansson, E.M.; Barrabino, A.; Odén, M.; Holmberg, K. Immobilization of lipase from Mucor miehei and Rhizopus oryzae into mesoporous silica–The effect of varied particle size and morphology. Colloids Surf. B Biointerfaces 2012, 100, 22–30. [Google Scholar] [CrossRef] [Green Version]
- Onoja, E.; Chandren, S.; Razak, F.I.A.; Wahab, R.A. Enzymatic synthesis of butyl butyrate by Candida rugosa lipase supported on magnetized-nanosilica from oil palm leaves: Process optimization, kinetic and thermodynamic study. J. Taiwan Inst. Chem. Eng. 2018, 91, 105–118. [Google Scholar] [CrossRef]
- Isah, A.A.; Mahat, N.A.; Jamalis, J.; Attan, N.; Zakaria, I.I.; Huyop, F.; Wahab, R.A. Synthesis of geranyl propionate in a solvent-free medium using Rhizomucor miehei lipase covalently immobilized on chitosan–graphene oxide beads. Prep. Biochem. Biotechnol. 2017, 47, 199–210. [Google Scholar] [CrossRef]
- Elias, N.; Chandren, S.; Attan, N.; Mahat, N.A.; Razak, F.I.A.; Jamalis, J.; Wahab, R.A. Structure and properties of oil palm-based nanocellulose reinforced chitosan nanocomposite for efficient synthesis of butyl butyrate. Carbohydr. Polym. 2017, 176, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Rahman, I.N.A.; Attan, N.; Mahat, N.A.; Jamalis, J.; Keyon, A.S.A.; Kurniawan, C.; Wahab, R.A. Statistical optimization and operational stability of Rhizomucor miehei lipase supported on magnetic chitosan/chitin nanoparticles for synthesis of pentyl valerate. Int. J. Biol. Macromol. 2018, 115, 680–695. [Google Scholar] [CrossRef] [PubMed]
- Rahman, I.N.A.; Wahab, R.A.; Mahat, N.A.; Jamalis, J.; Huri, M.A.M.; Kurniawan, C. Ternary blended chitosan/chitin/Fe3O4 nanosupport for lipase activation and stabilization. Arab. J. Sci. Eng. 2019, 44, 6327–6337. [Google Scholar] [CrossRef]
- Hussin, F.N.N.M.; Attan, N.; Wahab, R.A. Taguchi design-assisted immobilization of Candida rugosa lipase onto a ternary alginate/nanocellulose/montmorillonite composite: Physicochemical characterization, thermal stability and reusability studies. Enzym. Microb. Technol. 2020, 136, 109506. [Google Scholar] [CrossRef]
- Zhao, P.; Tian, L.; Li, X.; Ali, Z.; Zhang, B.; Zhang, H.; Zhang, Q. Effect of the structure and length of flexible chains on dendrimers grafted Fe3O4@SiO2/PAMAM magnetic nanocarriers for lipase immobilization. ACS Sustain. Chem. Eng. 2016, 4, 6382–6390. [Google Scholar] [CrossRef]
- Elias, N.; Wahab, R.A.; Jye, L.W.; Mahat, N.A.; Chandren, S.; Jamalis, J. Taguchi orthogonal design assisted immobilization of Candida rugosa lipase onto nanocellulose-silica reinforced polyethersulfone membrane: Physicochemical characterization and operational stability. Cellulose 2021, 28, 5669–5691. [Google Scholar] [CrossRef]
- Bhavsar, K.V.; Yadav, G.D. Process intensification by microwave irradiation in immobilized-lipase catalysis in solvent-free synthesis of ethyl valerate. Mol. Catal. 2018, 461, 34–39. [Google Scholar] [CrossRef]
- Cebrián-García, S.; Balu, A.M.; García, A.; Luque, R. Sol-gel immobilisation of lipases: Towards active and stable biocatalysts for the esterification of valeric acid. Molecules 2018, 23, 2283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khoobi, M.; Motevalizadeh, S.F.; Asadgol, Z.; Forootanfar, H.; Shafiee, A.; Faramarzi, M.A. Polyethyleneimine-modified superparamagnetic Fe3O4 nanoparticles for lipase immobilization: Characterization and application. Mater. Chem. Phys. 2015, 149, 77–86. [Google Scholar] [CrossRef]
- Moreira, W.C.; Elias, A.L.P.; Osório, W.R.; Padilha, G.S. Alternative method to improve the ethyl valerate yield using an immobilised Burkholderia cepacia lipase. J. Microencapsul. 2019, 36, 327–337. [Google Scholar] [CrossRef]
- Padilha, G.S.; Barros, M.D.; Alegre, R.M.; Tambourgi, E. Production of ethyl valerate from Burkholderia cepacia lipase immobilized in alginate. Chem. Eng. Trans. 2013, 32, 1063–1068. [Google Scholar] [CrossRef]
- Raghavendra, T.; Sayania, D.; Madamwar, D. Synthesis of the ‘green apple ester’ethyl valerate in organic solvents by Candida rugosa lipase immobilized in MBGs in organic solvents: Effects of immobilization and reaction parameters. J. Mol. Catal. B Enzym. 2010, 63, 31–38. [Google Scholar] [CrossRef]
- Bayramoğlu, G.; Hazer, B.; Altıntaş, B.; Arıca, M.Y. Covalent immobilization of lipase onto amine functionalized polypropylene membrane and its application in green apple flavor (ethyl valerate) synthesis. Process Biochem. 2011, 46, 372–378. [Google Scholar] [CrossRef]
- Ranjbari, N.; Razzaghi, M.; Fernandez-Lafuente, R.; Shojaei, F.; Satari, M.; Homaei, A. Improved features of a highly stable protease from Penaeus vannamei by immobilization on glutaraldehyde activated graphene oxide nanosheets. Int. J. Biol. Macromol. 2019, 130, 564–572. [Google Scholar] [CrossRef]
- Abd Manan, F.M.; Attan, N.; Zakaria, Z.; Keyon, A.S.A.; Wahab, R.A. Enzymatic esterification of eugenol and benzoic acid by a novel chitosan-chitin nanowhiskers supported Rhizomucor miehei lipase: Process optimization and kinetic assessments. Enzym. Microb. Technol. 2018, 108, 42–52. [Google Scholar] [CrossRef]
- Vakili, F.; Mojtabavi, S.; Imanparast, S.; Kianmehr, Z.; Forootanfar, H.; Faramarzi, M.A. Immobilization of lipase on the modified magnetic diatomite earth for effective methyl esterification of isoamyl alcohol to synthesize banana flavor. 3 Biotech 2020, 10, 447. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Abd Manan, F.M.; Attan, N.; Zakaria, Z.; Mahat, N.A.; Wahab, R.A. Insight into the Rhizomucor miehei lipase supported on chitosan-chitin nanowhiskers assisted esterification of eugenol to eugenyl benzoate. J. Biotechnol. 2018, 280, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, A. Reaction rates and selectivity in catalyst pores. In Advances in Catalysis; Academic Press: Cambridge, MA, USA, 1951; Volume 3, pp. 249–327. [Google Scholar] [CrossRef]
- Dhiman, S.; Srivastava, B.; Singh, G.; Khatri, M.; Arya, S.K. Immobilization of mannanase on sodium alginate-grafted-β-cyclodextrin: An easy and cost effective approach for the improvement of enzyme properties. Int. J. Biol. Macromol. 2020, 156, 1347–1358. [Google Scholar] [CrossRef]
- Yang, A.; Wang, Z.; Zhu, Y. Facile preparation and highly efficient sorption of magnetic composite graphene oxide/Fe3O4/GC for uranium removal. Sci. Rep. 2021, 11, 8440. [Google Scholar] [CrossRef]
- Xie, W.; Huang, M. Immobilization of Candida rugosa lipase onto graphene oxide Fe3O4 nanocomposite: Characterization and application for biodiesel production. Energy Convers. Manag. 2018, 159, 42–53. [Google Scholar] [CrossRef]
- Khan, Q.A.; Shaur, A.; Khan, T.A.; Joya, Y.F.; Awan, M.S. Characterization of reduced graphene oxide produced through a modified Hoffman method. Cogent Chem. 2017, 3, 1298980. [Google Scholar] [CrossRef]
- Alam, S.N.; Sharma, N.; Kumar, L. Synthesis of graphene oxide (GO) by modified hummers method and its thermal reduction to obtain reduced graphene oxide (rGO). Graphene 2017, 6, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Alves, M.D.; Aracri, F.M.; Cren, É.C.; Mendes, A.A. Isotherm, kinetic, mechanism and thermodynamic studies of adsorption of a microbial lipase on a mesoporous and hydrophobic resin. Chem. Eng. Sci. 2017, 311, 1–12. [Google Scholar] [CrossRef]
- Qilong, S.; Lei, S.; Yingying, C.; Wei, Y.; Sijun, X.; Tao, J.; Guoqiu, Y. Fe3O4-intercalated reduced graphene oxide nanocomposites with enhanced microwave absorption properties. Ceram. Int. 2019, 45, 18298–18305. [Google Scholar] [CrossRef]
- Noma, S.A.A.; Ulu, A.; Koytepe, S.; Ateş, B. Preparation and characterization of amino and carboxyl functionalized core-shell Fe3O4/SiO2 for L-asparaginase immobilization: A comparison study. Biocatal. Biotransform. 2020, 38, 392–404. [Google Scholar] [CrossRef]
- Vasić, K.; Knez, Ž.; Konstantinova, E.A.; Kokorin, A.I.; Gyergyek, S.; Leitgeb, M. Structural and magnetic characteristics of carboxymethyl dextran coated magnetic nanoparticles: From characterization to immobilization application. React. Funct. Polym. 2020, 148, 104481. [Google Scholar] [CrossRef]
- Schnepp, Z.; Wimbush, S.C.; Antonietti, M.; Giordano, C. Synthesis of highly magnetic iron carbide nanoparticles via a biopolymer route. Chem. Mater. 2010, 22, 5340–5344. [Google Scholar] [CrossRef]
- Elias, N.; Wahab, R.A.; Chandren, S.; Razak, F.I.A.; Jamalis, J. Effect of operative variables and kinetic study of butyl butyrate synthesis by Candida rugosa lipase activated by chitosan-reinforced nanocellulose derived from raw oil palm leaves. Enzym. Microb. Technol. 2019, 130, 109367. [Google Scholar] [CrossRef]
- Zhao, J.; Ma, M.; Yan, X.; Wan, D.; Zeng, Z.; Yu, P.; Gong, D. Immobilization of lipase on β-cyclodextrin grafted and aminopropyl-functionalized chitosan/Fe3O4 magnetic nanocomposites: An innovative approach to fruity flavor esters esterification. Food Chem. 2022, 366, 130616. [Google Scholar] [CrossRef]
- Bayramoglu, G.; Salih, B.; Arica, M.Y. Catalytic activity of immobilized chymotrypsin on hybrid silica-magnetic biocompatible particles and its application in peptide synthesis. Appl. Biochem. Biotechnol. 2020, 190, 1224–1241. [Google Scholar] [CrossRef]
- Taghizadeh, T.; Ameri, A.; Talebian-Kiakalaieh, A.; Mojtabavi, S.; Ameri, A.; Forootanfar, H.; Faramarzi, M.A. Lipase@zeolitic imidazolate framework ZIF-90: A highly stable and recyclable biocatalyst for the synthesis of fruity banana flavour. Int. J. Biol. 2021, 166, 1301–1311. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, W.; Zhao, L.; Liu, H. Degradation of phenol with Horseradish Peroxidase immobilized on ZnO nanocrystals under combined irradiation of microwaves and ultrasound. Desalin. Water Treat. 2016, 57, 24406–24416. [Google Scholar] [CrossRef]
- Jaiswal, K.S.; Rathod, V.K. Green synthesis of amyl levulinate using lipase in the solvent free system: Optimization, mechanism and thermodynamics studies. Catal. Today 2021, 375, 120–131. [Google Scholar] [CrossRef]
- Bodakowska-Boczniewicz, J.; Garncarek, Z. Immobilization of naringinase from aspergillus niger on a magnetic polysaccharide carrier. Molecules 2020, 25, 2731. [Google Scholar] [CrossRef]
- Liu, T.; Liu, Y.; Wang, X.; Li, Q.; Wang, J.; Yan, Y. Improving catalytic performance of Burkholderia cepacia lipase immobilized on macroporous resin NKA. J. Mol. Catal. B Enzym. 2011, 71, 45–50. [Google Scholar] [CrossRef]
- Agrawal, D.C.; Yadav, A.; Kesarwani, R.; Srivastava, O.N.; Kayastha, A.M. Immobilization of fenugreek β-amylase onto functionalized graphene quantum dots (GQDs) using Box-Behnken design: Its biochemical, thermodynamic and kinetic studies. Int. J. Biol. Macromol. 2020, 144, 170–182. [Google Scholar] [CrossRef] [PubMed]
- Elias, N.; Wahab, R.A.; Chandren, S.; Lau, W.J. Performance of Candida rugosa lipase supported on nanocellulose-silica-reinforced polyethersulfone membrane for the synthesis of pentyl valerate: Kinetic, thermodynamic and regenerability studies. Mol. Catal. 2021, 514, 111852. [Google Scholar] [CrossRef]
- Ni, J.; Mahdavi, B.; Ghezi, S. Chemical composition, antimicrobial, hemolytic, and antiproliferative activity of essential oils from Ephedra intermedia Schrenk & Mey. J. Essent. Oil Bear. Plants 2019, 22, 1562–1570. [Google Scholar] [CrossRef]













| Biocatalyst | R2 | R2 | ||
|---|---|---|---|---|
| Free C. rugosa lipase | 15.26 | 0.9855 | 27.60 | 0.9714 |
| CRL/SiO2/Fe3O4/GO | 13.87 | 0.9914 | 32.32 | 0.9771 |
| Parameter | Biocatalyst | Temperature | ||||
|---|---|---|---|---|---|---|
| 45 °C | 50 °C | 55 °C | 60 °C | 70 °C | ||
| (min−1) | Free CRL | 0.0055 | 0.0060 | 0.0071 | 0.0087 | 0.0131 |
| CRLSiO2/Fe3O4/GO | 0.0042 | 0.0048 | 0.0053 | 0.0061 | 0.0091 | |
| (min) | Free CRL | 126.03 | 115.52 | 97.63 | 79.67 | 52.91 |
| CRLSiO2/Fe3O4/GO | 165.04 | 144.41 | 130.78 | 113.63 | 76.17 | |
| SF | CRLSiO2/Fe3O4/GO | 1.310 | 1.250 | 1.340 | 1.426 | 1.450 |
| D-value (min) | Free CRL | 418.65 | 383.76 | 324.31 | 264.66 | 175.77 |
| CRLSiO2/Fe3O4/GO | 548.23 | 479.71 | 434.45 | 377.47 | 253.03 | |
| (kJ mol−1) | Free CRL | 24.96 | 24.92 | 24.87 | 24.83 | 24.75 |
| CRLSiO2/Fe3O4/GO | 29.67 | 29.63 | 29.59 | 29.55 | 29.46 | |
| (kJ mol−1) | Free CRL | 13.79 | 13.76 | 13.51 | 13.25 | 12.35 |
| CRLSiO2/Fe3O4/GO | 14.50 | 14.36 | 14.31 | 14.23 | 13.39 | |
| (J mol−1K−1) | Free CRL | 35.11 | 34.54 | 34.62 | 34.76 | 36.14 |
| CRLSiO2/Fe3O4/GO | 47.68 | 47.25 | 46.56 | 45.99 | 46.83 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jacob, A.G.; Wahab, R.A.; Misson, M. Operational Stability, Regenerability, and Thermodynamics Studies on Biogenic Silica/Magnetite/Graphene Oxide Nanocomposite-Activated Candida rugosa Lipase. Polymers 2021, 13, 3854. https://doi.org/10.3390/polym13213854
Jacob AG, Wahab RA, Misson M. Operational Stability, Regenerability, and Thermodynamics Studies on Biogenic Silica/Magnetite/Graphene Oxide Nanocomposite-Activated Candida rugosa Lipase. Polymers. 2021; 13(21):3854. https://doi.org/10.3390/polym13213854
Chicago/Turabian StyleJacob, Adikwu Gowon, Roswanira Abdul Wahab, and Mailin Misson. 2021. "Operational Stability, Regenerability, and Thermodynamics Studies on Biogenic Silica/Magnetite/Graphene Oxide Nanocomposite-Activated Candida rugosa Lipase" Polymers 13, no. 21: 3854. https://doi.org/10.3390/polym13213854