Interfacial Behavior of Solid- and Liquid-like Polyelectrolyte Complexes as a Function of Charge Stoichiometry
Abstract
:1. Introduction
2. Experimental Section
3. Results and Discussion
3.1. Surface Tension of Individual Polyelectrolyte Solutions
3.2. Dynamic Light Scattering Analysis and Zeta Potential of PECs
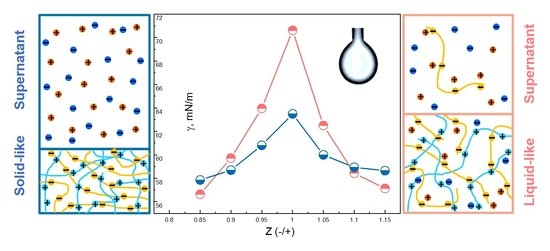
3.3. Surface Tension of PEC Dispersions
3.4. Interfacial Tension between the Dilute and Concentrated Coacervate Phase
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wilts, E.M.; Herzberger, J.; Long, T.E. Addressing water scarcity: Cationic polyelectrolytes in water treatment and purification. Polym. Int. 2018, 67, 799–814. [Google Scholar] [CrossRef]
- Schnell, C.N.; Tarrés, Q.; Galván, M.V.; Mocchiutti, P.; Delgado-Aguilar, M.; Zanuttini, M.A.; Mutjé, P. Polyelectrolyte complexes for assisting the application of lignocellulosic micro/nanofibers in papermaking. Cellulose 2018, 25, 6083–6092. [Google Scholar] [CrossRef]
- Schmitt, C.; Turgeon, S.L. Protein/polysaccharide complexes and coacervates in food systems. Adv. Colloid Interface Sci. 2011, 167, 63–70. [Google Scholar] [CrossRef]
- Meka, V.S.; Sing, M.K.G.; Pichika, M.R.; Nali, S.R.; Kolapalli, V.R.M.; Kesharwani, P. A comprehensive review on polyelectrolyte complexes. Drug Discov. Today 2017, 22, 1697–1706. [Google Scholar] [CrossRef]
- Llamas, S.; Guzman, E.; Ortega, F.; Baghdadli, N.; Cazeneuve, C.; Rubio, R.G.; Luengo, G.S. Adsorption of polyelectrolytes and polyelectrolytes-surfactant mixtures at surfaces: A physico-chemical approach to a cosmetic challenge. Adv. Colloid Interface Sci. 2015, 222, 461–487. [Google Scholar] [CrossRef] [PubMed]
- Shakshi, R.; Pramod Kumar, S.; Rishabha, M. Pharmaceutical and Tissue Engineering Applications of Polyelectrolyte Complexes. Curr. Smart Mater. 2018, 3, 21–31. [Google Scholar] [CrossRef]
- Buriuli, M.; Verma, D. Polyelectrolyte Complexes (PECs) for Biomedical Applications. In Advances in Biomaterials for Biomedical Applications; Tripathi, A., Melo, J.S., Eds.; Springer: Singapore, 2017; pp. 45–93. [Google Scholar]
- Lebovka, N.I. Aggregation of Charged Colloidal Particles. In Polyelectrolyte Complexes in the Dispersed and Solid State I: Principles and Theory; Advances in Polymer Science; Müller, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 57–96. [Google Scholar]
- Fu, J.; Schlenoff, J.B. Driving Forces for Oppositely Charged Polyion Association in Aqueous Solutions: Enthalpic, Entropic, but Not Electrostatic. J. Am. Chem. Soc. 2016, 138, 980–990. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Schlenoff, J.B. The Polyelectrolyte Complex/Coacervate Continuum. Macromolecules 2014, 47, 3108–3116. [Google Scholar] [CrossRef]
- Liu, X.; Chapel, J.P.; Schatz, C. Structure, thermodynamic and kinetic signatures of a synthetic polyelectrolyte coacervating system. Adv. Colloid Interface Sci. 2017, 239, 178–186. [Google Scholar] [CrossRef]
- Liu, X.; Haddou, M.; Grillo, I.; Mana, Z.; Chapel, J.P.; Schatz, C. Early stage kinetics of polyelectrolyte complex coacervation monitored through stopped-flow light scattering. Soft Matter 2016, 12, 9030–9038. [Google Scholar] [CrossRef]
- Vitorazi, L.; Ould-Moussa, N.; Sekar, S.; Fresnais, J.; Loh, W.; Chapel, J.P.; Berret, J.F. Evidence of a two-step process and pathway dependency in the thermodynamics of poly(diallyldimethylammonium chloride)/poly(sodium acrylate) complexation. Soft Matter 2014, 10, 9496–9505. [Google Scholar] [CrossRef] [PubMed]
- Ishimuro, Y.; Ueberreiter, K. The surface tension of poly(acrylic acid) in aqueous solution. Colloid Polym. Sci. 1980, 258, 928–931. [Google Scholar] [CrossRef]
- Theodoly, O.; Ober, R.; Williams, C.E. Adsorption of hydrophobic polyelectrolytes at the air/water interface: Conformational effect and history dependence. Eur. Phys. J. E 2001, 5, 51–58. [Google Scholar] [CrossRef]
- Noskov, B.A.; Nuzhnov, S.N.; Loglio, G.; Miller, R. Dynamic Surface Properties of Sodium Poly(styrenesulfonate) Solutions. Macromolecules 2004, 37, 2519–2526. [Google Scholar] [CrossRef]
- Noskov, B.A.; Bilibin, A.Y.; Lezov, A.V.; Loglio, G.; Filippov, S.K.; Zorin, I.M.; Miller, R. Dynamic surface elasticity of polyelectrolyte solutions. Colloids Surf. A Physicochem. Eng. Asp. 2007, 298, 115–122. [Google Scholar] [CrossRef]
- Yim, H.; Kent, M.; Matheson, A.; Ivkov, R.; Satija, S.; Majewski, J.; Smith, G.S. Adsorption of Poly(styrenesulfonate) to the Air Surface of Water by Neutron Reflectivity. Macromolecules 2000, 33, 6126–6133. [Google Scholar] [CrossRef]
- Yim, H.; Kent, M.S.; Matheson, A.; Stevens, M.J.; Ivkov, R.; Satija, S.; Majewski, J.; Smith, G.S. Adsorption of Sodium Poly(styrenesulfonate) to the Air Surface of Water by Neutron and X-ray Reflectivity and Surface Tension Measurements: Polymer Concentration Dependence. Macromolecules 2002, 35, 9737–9747. [Google Scholar] [CrossRef]
- Owiwe, M.T.; Ayyad, A.H.; Takrori, F.M. Surface tension of the oppositely charged sodium poly(styrene sulfonate) /benzyldimethylhexadecylammonium chloride and sodium poly(styrene sulfonate)/polyallylamine hydrochloride mixtures. Colloid Polym. Sci. 2020, 298, 1197–1204. [Google Scholar] [CrossRef]
- Dickhaus, B.N.; Priefer, R. Determination of polyelectrolyte pKa values using surface-to-air tension measurements. Colloids Surf. A Physicochem. Eng. Asp. 2016, 488, 15–19. [Google Scholar] [CrossRef]
- Okubo, T.; Kobayashi, K. Surface Tension of Biological Polyelectrolyte Solutions. J. Colloid Interface Sci. 1998, 205, 433–442. [Google Scholar] [CrossRef]
- Ríos, H.E.; González-Navarrete, J.; Vargas, V.; Urzúa, M.D. Surface properties of cationic polyelectrolytes hydrophobically modified. Colloids Surf. A Physicochem. Eng. Asp. 2011, 384, 262–267. [Google Scholar] [CrossRef]
- Millet, F.; Nedyalkov, M.; Renard, B.; Perrin, P.; Lafuma, F.; Benattar, J.-J. Adsorption of Hydrophobically Modified Poly(acrylic acid) Sodium Salt at the Air/Water Interface by Combined Surface Tension and X-ray Reflectivity Measurements. Langmuir 1999, 15, 2112–2119. [Google Scholar] [CrossRef]
- Taylor, D.J.; Thomas, R.K.; Penfold, J. Polymer/surfactant interactions at the air/water interface. Adv. Colloid Interface Sci. 2007, 132, 69–110. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Brettmann, B. Intermolecular Interactions in Polyelectrolyte and Surfactant Complexes in Solution. Polymers 2018, 11, 51. [Google Scholar] [CrossRef]
- La Mesa, C. Polymer–surfactant and protein–surfactant interactions. J. Colloid Interface Sci. 2005, 286, 148–157. [Google Scholar] [CrossRef]
- Budhathoki, M.; Barnee, S.H.R.; Shiau, B.-J.; Harwell, J.H. Improved oil recovery by reducing surfactant adsorption with polyelectrolyte in high saline brine. Colloids Surf. A Physicochem. Eng. Asp. 2016, 498, 66–73. [Google Scholar] [CrossRef]
- Shulevich, Y.V.; Nguyen, T.H.; Tutaev, D.S.; Navrotskii, A.V.; Novakov, I.A. Purification of fat-containing wastewater using polyelectrolyte–surfactant complexes. Sep. Purif. Technol. 2013, 113, 18–23. [Google Scholar] [CrossRef]
- Mirtič, J.; Paudel, A.; Laggner, P.; Hudoklin, S.; Kreft, M.E.; Kristl, J. Polyelectrolyte–surfactant–complex nanoparticles as a delivery platform for poorly soluble drugs: A case study of ibuprofen loaded cetylpyridinium-alginate system. Int. J. Pharm. 2020, 580, 119199. [Google Scholar] [CrossRef] [PubMed]
- Grządka, E.; Matusiak, J.; Stankevič, M. Interactions between fluorocarbon surfactants and polysaccharides. J. Mol. Liq. 2019, 283, 81–90. [Google Scholar] [CrossRef]
- Bago Rodriguez, A.M.; Binks, B.P.; Sekine, T. Emulsion stabilisation by complexes of oppositely charged synthetic polyelectrolytes. Soft Matter 2018, 14, 239–254. [Google Scholar] [CrossRef]
- Bago Rodriguez, A.M.; Binks, B.P.; Sekine, T. Novel stabilisation of emulsions by soft particles: Polyelectrolyte complexes. Faraday Discuss. 2016, 191, 255–285. [Google Scholar] [CrossRef] [PubMed]
- Generalova, A.N.; Marchenko, S.B.; Gorokhova, I.V.; Miller, R.; Gurevich, I.V.; Tsarkova, M.S.; Maksimov, V.I.; Zaitsev, S.Y. Advantages of interfacial tensiometry for studying the interactions of biologically active compounds. Colloids Surf. A Physicochem. Eng. Asp. 2007, 298, 88–93. [Google Scholar] [CrossRef]
- Nekrasova, T.N.; Anufriyeva, Y.V.; Yel’yashevich, A.M.; Ptitsyn, O.B. Potentiometric titration of polyacrylic acid, polymethacrylic acid and poly-l-glutamic acid. Polym. Sci. U.S.S.R. 1965, 7, 1008–1018. [Google Scholar] [CrossRef]
- Chen, P.; Kwok, D.Y.; Prokop, R.M.; del Rio, O.I.; Susnar, S.S.; Neumann, A.W. Axisymmetric Drop Shape Analysis (ADSA) and its Applications. In Studies in Interface Science; MÖbius, D., Miller, R., Eds.; Elsevier: Amsterdam, The Netherlands, 1998; Volume 6, pp. 61–138. [Google Scholar]
- Aarts, D.G.A.L.; Wiel, J.H.v.d.; Lekkerkerker, H.N.W. Interfacial dynamics and the static profile near a single wall in a model colloid polymer mixture. J. Phys. Condens. Matter 2002, 15, S245–S250. [Google Scholar] [CrossRef]
- Batchelor, G.K. The Physical Properties of Fluids. In An Introduction to Fluid Dynamics; Cambridge Mathematical Library; Cambridge University Press: Cambridge, UK, 2000; pp. 1–70. [Google Scholar]
- Manning, G.S. Limiting Laws and Counterion Condensation in Polyelectrolyte Solutions I. Colligative Properties. J. Chem. Phys. 1969, 51, 924–933. [Google Scholar] [CrossRef]
- Oosawa, F. Polyelectrolytes; M. Dekker: New York, NY, USA, 1971. [Google Scholar]
- Sedlak, M. What can be seen by static and dynamic light scattering in polyelectrolyte solutions and mixtures? Langmuir 1999, 15, 4045–4051. [Google Scholar] [CrossRef]
- Kabanov, V.A.; Zezin, A.B. A new class of complex water-soluble polyelectrolytes. Die Makromol. Chem. 1984, 6, 259–276. [Google Scholar] [CrossRef]
- Muthukumar, M. Ordinary–extraordinary transition in dynamics of solutions of charged macromolecules. Proc. Natl. Acad. Sci. USA 2016, 113, 12627–12632. [Google Scholar] [CrossRef] [PubMed]
- Mende, M.; Schwarz, S.; Zschoche, S.; Petzold, G.; Janke, A. Influence of the Hydrophobicity of Polyelectrolytes on Polyelectrolyte Complex Formation and Complex Particle Structure and Shape. Polymers 2011, 3, 1363–1376. [Google Scholar] [CrossRef]
- Zhang, R.; Shklovskii, B.I. Phase diagram of solution of oppositely charged polyelectrolytes. Phys. A Stat. Mech. Appl. 2005, 352, 216–238. [Google Scholar] [CrossRef]
- Veis, A.; Aranyi, C. Phase separation in polyelectrolyte systems. I. Complex coacervates of gelatin. J. Phys. Chem. 1960, 64, 1203–1210. [Google Scholar] [CrossRef]
- Kizilay, E.; Kayitmazer, A.B.; Dubin, P.L. Complexation and coacervation of polyelectrolytes with oppositely charged colloids. Adv. Colloid Interface Sci. 2011, 167, 24–37. [Google Scholar] [CrossRef]
- Manga, M.S.; Hunter, T.N.; Cayre, O.J.; York, D.W.; Reichert, M.D.; Anna, S.L.; Walker, L.M.; Williams, R.A.; Biggs, S.R. Measurements of Submicron Particle Adsorption and Particle Film Elasticity at Oil–Water Interfaces. Langmuir 2016, 32, 4125–4133. [Google Scholar] [CrossRef] [PubMed]
- Michaels, A.S.; Mir, L.; Schneider, N.S. A Conductometric Study of Polycation—Polyanion Reactions in Dilute Aqueous Solution. J. Phys. Chem. 1965, 69, 1447–1455. [Google Scholar] [CrossRef]
- Spruijt, E.; Sprakel, J.; Cohen Stuart, M.A.; van der Gucht, J. Interfacial tension between a complex coacervate phase and its coexisting aqueous phase. Soft Matter 2010, 6, 172–178. [Google Scholar] [CrossRef]
- Ali, S.; Prabhu, V.M. Characterization of the Ultralow Interfacial Tension in Liquid-Liquid Phase Separated Polyelectrolyte Complex Coacervates by the Deformed Drop Retraction Method. Macromolecules 2019, 52, 7495–7502. [Google Scholar] [CrossRef]
- Prabhu, V.M. Interfacial tension in polyelectrolyte systems exhibiting associative liquid–liquid phase separation. Curr. Opin. Colloid Interface Sci. 2021, 53, 101422. [Google Scholar] [CrossRef]
- Priftis, D.; Farina, R.; Tirrell, M. Interfacial Energy of Polypeptide Complex Coacervates Measured via Capillary Adhesion. Langmuir 2012, 28, 8721–8729. [Google Scholar] [CrossRef]
- Qin, J.; Priftis, D.; Farina, R.; Perry, S.L.; Leon, L.; Whitmer, J.; Hoffmann, K.; Tirrell, M.; de Pablo, J.J. Interfacial Tension of Polyelectrolyte Complex Coacervate Phases. ACS Macro Lett. 2014, 3, 565–568. [Google Scholar] [CrossRef]
- Vis, M.; Blokhuis, E.M.; Erné, B.H.; Tromp, R.H.; Lekkerkerker, H.N.W. Interfacial Tension of Phase-Separated Polydisperse Mixed Polymer Solutions. J. Phys. Chem. B 2018, 122, 3354–3362. [Google Scholar] [CrossRef]
- Atefi, E.; Mann, J.A.; Tavana, H. Ultralow Interfacial Tensions of Aqueous Two-Phase Systems Measured Using Drop Shape. Langmuir 2014, 30, 9691–9699. [Google Scholar] [CrossRef] [PubMed]
- Gucht, J.v.d.; Spruijt, E.; Lemmers, M.; Cohen Stuart, M.A. Polyelectrolyte complexes: Bulk phases and colloidal systems. J. Colloid Interface Sci. 2011, 361, 407–422. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Zacharia, N.S. Protein encapsulation via polyelectrolyte complex coacervation: Protection against protein denaturation. J. Chem. Phys. 2018, 149, 163326. [Google Scholar] [CrossRef] [PubMed]
- Douliez, J.-P.; Martin, N.; Beneyton, T.; Eloi, J.-C.; Chapel, J.-P.; Navailles, L.; Baret, J.-C.; Mann, S.; Béven, L. Preparation of Swellable Hydrogel-Containing Colloidosomes from Aqueous Two-Phase Pickering Emulsion Droplets. Angew. Chem. Int. Ed. 2018, 57, 7780–7784. [Google Scholar] [CrossRef] [PubMed]
- Esquena, J. Water-in-water (W/W) emulsions. Curr. Opin. Colloid Interface Sci. 2016, 25, 109–119. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Fauquignon, M.; Haddou, M.; Schatz, C.; Chapel, J.-P. Interfacial Behavior of Solid- and Liquid-like Polyelectrolyte Complexes as a Function of Charge Stoichiometry. Polymers 2021, 13, 3848. https://doi.org/10.3390/polym13213848
Li H, Fauquignon M, Haddou M, Schatz C, Chapel J-P. Interfacial Behavior of Solid- and Liquid-like Polyelectrolyte Complexes as a Function of Charge Stoichiometry. Polymers. 2021; 13(21):3848. https://doi.org/10.3390/polym13213848
Chicago/Turabian StyleLi, Hongwei, Martin Fauquignon, Marie Haddou, Christophe Schatz, and Jean-Paul Chapel. 2021. "Interfacial Behavior of Solid- and Liquid-like Polyelectrolyte Complexes as a Function of Charge Stoichiometry" Polymers 13, no. 21: 3848. https://doi.org/10.3390/polym13213848