Facile Modification of NF Membrane by Multi-Layer Deposition of Polyelectrolytes for Enhanced Fouling Resistance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
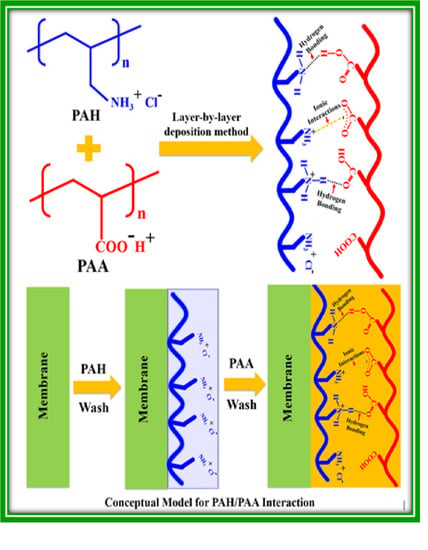
2.2. Preparation of the Polyelectrolyte LBL Assembled Membranes
2.3. Membrane Characterization
2.4. Nanofiltration Performance of the Membranes
2.5. Evaluation of Antifouling Performance of the Membranes
3. Results and Discussion
3.1. Membrane Assembly and the Conceptual Model
3.2. The Surface Functionalities
3.3. Morphological Features of the Membranes
3.4. Surface Roughness and Wettability
3.5. Thermogravimetric Analysis of the Membranes
3.6. Evaluation of Membrane Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liang, C.Z.; Chung, T.S.; Lai, J.Y. A review of polymeric composite membranes for gas separation and energy production. Prog. Polym. Sci. 2019, 97, 101141. [Google Scholar] [CrossRef]
- Zuo, H.-R.; Shi, P.; Duan, M. A review on thermally stable membranes for water treatment: Material, fabrication, and application. Sep. Purif. Technol. 2019, 116223. [Google Scholar] [CrossRef]
- Tian, J.; Zhao, X.; Gao, S.; Wang, X.; Zhang, R. Progress in research and application of nanofiltration (nf) technology for brackish water treatment. Membranes 2021, 11, 662. [Google Scholar] [CrossRef]
- Salehi, F. Current and future applications for nanofiltration technology in the food processing. Food Bioprod. Process. 2014, 92, 161–177. [Google Scholar] [CrossRef]
- Van der Bruggen, B.; Mänttäri, M.; Nyström, M. Drawbacks of applying nanofiltration and how to avoid them: A review. Sep. Purif. Technol. 2008, 63, 251–263. [Google Scholar] [CrossRef]
- Kaur, S.; Ma, Z.; Gopal, R.; Singh, G.; Ramakrishna, S.; Matsuura, T. Plasma-induced graft copolymerization of poly(methacrylic acid) on electrospun poly(vinylidene fluoride) nanofiber membrane. Langmuir 2007, 23, 13085–13092. [Google Scholar] [CrossRef]
- Sarkar, A.; Carver, P.I.; Zhang, T.; Merrington, A.; Bruza, K.J.; Rousseau, J.L.; Keinath, S.E.; Dvornic, P.R. Dendrimer-based coatings for surface modification of polyamide reverse osmosis membranes. J. Memb. Sci. 2010, 349, 421–428. [Google Scholar] [CrossRef]
- Kim, H.I.; Kim, S.S. Plasma treatment of polypropylene and polysulfone supports for thin film composite reverse osmosis membrane. J. Memb. Sci. 2006, 286, 193–201. [Google Scholar] [CrossRef]
- Nasef, M.M.; Gupta, B.; Shameli, K.; Verma, C.; Ali, R.R.; Ting, T.M. Engineered Bioactive Polymeric Surfaces by Radiation Induced Graft Copolymerization: Strategies and Applications. Polymers 2021, 13, 3102. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Belfort, G. Surface modification of poly(ether sulfone) ultrafiltration membranes by low-temperature plasma-induced graft polymerization. J. Appl. Polym. Sci. 1999, 72, 1699–1711. [Google Scholar] [CrossRef]
- Kong, C.; Shintani, T.; Tsuru, T. “Pre-seeding”-assisted synthesis of a high performance polyamide-zeolite nanocomposite membrane for water purification. New J. Chem. 2010, 34, 2101–2104. [Google Scholar] [CrossRef]
- Syahirah Zakria, H.; Dzarfan Othman, M.H.; Kamaludin, R.; Kadir, S.H.S.A.; Agustiono Kurniawan, T.; Jilani, A. Immobilization techniques of a photocatalyst into and onto a polymer membrane for photocatalytic activity. RSC Adv. 2021, 11, 6985–7014. [Google Scholar] [CrossRef]
- Dubas, S.T.; Farhat, T.R.; Schlenoff, J.B. Multiple membranes from “true” polyelectrolyte multilayers. J. Am. Chem. Soc. 2001, 123, 5368–5369. [Google Scholar] [CrossRef]
- Petrila, L.-M.; Bucatariu, F.; Mihai, M.; Teodosiu, C. Polyelectrolyte Multilayers: An Overview on Fabrication, Properties, and Biomedical and Environmental Applications. Materials 2021, 14, 4152. [Google Scholar] [CrossRef]
- Saqib, J.; Aljundi, I.H. Membrane fouling and modification using surface treatment and layer-by-layer assembly of polyelectrolytes: State-of-the-art review. J. Water Process Eng. 2016, 11, 68–87. [Google Scholar] [CrossRef]
- Liu, Z.; An, X.; Dong, C.; Zheng, S.; Mi, B.; Hu, Y. Modification of thin film composite polyamide membranes with 3D hyperbranched polyglycerol for simultaneous improvement in their filtration performance and antifouling properties. J. Mater. Chem. A 2017, 5, 23190–23197. [Google Scholar] [CrossRef]
- Helali, N.; Shamaei, L.; Rastgar, M.; Sadrzadeh, M. Development of layer-by-layer assembled polyamide-imide membranes for oil sands produced water treatment. Sci. Rep. 2021, 11, 8098. [Google Scholar] [CrossRef] [PubMed]
- Charinpanitkul, T.; Suthabanditpong, W.; Watanabe, H.; Shirai, T.; Faungnawakij, K.; Viriya-empikul, N.; Fuji, M. Improved hydrophilicity of zinc oxide-incorporated layer-by-layer polyelectrolyte film fabricated by dip coating method. J. Ind. Eng. Chem. 2012, 18, 1441–1445. [Google Scholar] [CrossRef]
- Elahi, M.F.; Guan, G.; Wang, L.; King, M.W. Influence of layer-by-layer polyelectrolyte deposition and EDC/NHS activated heparin immobilization onto silk fibroin fabric. Materials 2014, 7, 2956–2977. [Google Scholar] [CrossRef] [Green Version]
- Kwon, Y.N.; Hong, S.; Choi, H.; Tak, T. Surface modification of a polyamide reverse osmosis membrane for chlorine resistance improvement. J. Memb. Sci. 2012, 415–416, 192–198. [Google Scholar] [CrossRef]
- Gu, J.E.; Lee, S.; Stafford, C.M.; Lee, J.S.; Choi, W.; Kim, B.Y.; Baek, K.Y.; Chan, E.P.; Chung, J.Y.; Bang, J.; et al. Molecular layer-by-layer assembled thin-film composite membranes for water desalination. Adv. Mater. 2013, 25, 4778–4782. [Google Scholar] [CrossRef] [PubMed]
- Ishigami, T.; Amano, K.; Fujii, A.; Ohmukai, Y.; Kamio, E.; Maruyama, T.; Matsuyama, H. Fouling reduction of reverse osmosis membrane by surface modification via layer-by-layer assembly. Sep. Purif. Technol. 2012, 99, 1–7. [Google Scholar] [CrossRef]
- Tripathi, B.P.; Dubey, N.C.; Stamm, M. Functional polyelectrolyte multilayer membranes for water purification applications. J. Hazard. Mater. 2013, 252–253, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.M.; Zuo, J.; Tang, S.H.; Wei, S.; Chung, T.S. Layer-by-layer (LbL) polyelectrolyte membrane with NexarTM polymer as a polyanion for pervaporation dehydration of ethanol. Sep. Purif. Technol. 2015, 140, 13–22. [Google Scholar] [CrossRef]
- Mu, S.; Wang, S.; Liang, S.; Xiao, K.; Fan, H.; Han, B.; Liu, C.; Wang, X.; Huang, X. Effect of the relative degree of foulant “hydrophobicity” on membrane fouling. J. Memb. Sci. 2019, 570–571, 1–8. [Google Scholar] [CrossRef]
- Hou, Q.; Wang, X.; Ragauskas, A.J. Dynamic self-assembly of polyelectrolyte composite nanomaterial film. Polymers 2019, 11, 1258. [Google Scholar] [CrossRef] [Green Version]
- Hadj Lajimi, R.; Ferjani, E.; Roudesli, M.S.; Deratani, A. Effect of LbL surface modification on characteristics and performances of cellulose acetate nanofiltration membranes. Desalination 2011, 266, 78–86. [Google Scholar] [CrossRef]
- Farhat, T.; Yassin, G.; Dubas, S.T.; Schlenoff, J.B. Water and ion pairing in polyelectrolyte multilayers. Langmuir 1999, 15, 6621–6623. [Google Scholar] [CrossRef]
- Svensson, O.; Lindh, L.; Cárdenas, M.; Arnebrant, T. Layer-by-layer assembly of mucin and chitosan-Influence of surface properties, concentration and type of mucin. J. Colloid Interface Sci. 2006, 299, 608–616. [Google Scholar] [CrossRef]
- Rmaile, H.H.; Schlenoff, J.B. “Internal pKa’s” in polyelectrolyte multilayers: Coupling protons and salt. Langmuir 2002, 18, 8263–8265. [Google Scholar] [CrossRef]
- Van Der Bruggen, B.; Koninckx, A.; Vandecasteele, C. Separation of monovalent and divalent ions from aqueous solution by electrodialysis and nanofiltration. Water Res. 2004, 38, 1347–1353. [Google Scholar] [CrossRef]
- Shao, W.; Liu, C.; Yu, T.; Xiong, Y.; Hong, Z.; Xie, Q. Constructing Positively Charged Thin-Film Nanocomposite Nanofiltration Membranes with Enhanced Performance. Polymers 2020, 12, 2526. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Zhang, S.; Ma, H.; Shao, W.; Gong, X.; Hong, Z. A Novel Thin-Film Nanocomposite Nanofiltration Membrane by Incorporating 3D Hyperbranched Polymer Functionalized 2D Graphene Oxide. Polymers 2018, 10, 1253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ou, C.; Li, S.; Wang, Z.; Qin, J.; Wang, Q.; Liao, Z.; Li, J. Organic Nanobowls Modified Thin Film Composite Membrane for Enhanced Purification Performance toward Different Water Resources. Membranes 2021, 11, 350. [Google Scholar] [CrossRef] [PubMed]
- An, Q.; Li, F.; Ji, Y.; Chen, H. Influence of polyvinyl alcohol on the surface morphology, separation and anti-fouling performance of the composite polyamide nanofiltration membranes. J. Memb. Sci. 2011, 367, 158–165. [Google Scholar] [CrossRef]
- Akbari, A.; Derikvandi, Z.; Mojallali Rostami, S.M. Influence of chitosan coating on the separation performance, morphology and anti-fouling properties of the polyamide nanofiltration membranes. J. Ind. Eng. Chem. 2015, 28, 268–276. [Google Scholar] [CrossRef]











| Membrane | Water Contact Angle (°) | Membrane Testing Conditions | Flux (LMH) | Salts Rejection (%) | Antifouling Performance | Reference |
|---|---|---|---|---|---|---|
| TFN-PEI-GO-20 NF membrane | ~35 | 2000 ppm Na2SO4; 5 bar; 25 °C | 70.3 | 91% | Better than pristine membrane | [32] |
| TFN-GHC-60 NF membrane | ~27 | 2000 ppm Na2SO4; 6 bar; 25 °C | 48.0 | >95% | Better than pristine membrane | [33] |
| Organic nanobowl containing TFN NF Membrane | ~55 | 1000 ppm Na2SO4; 6 bar; 25 °C | 100 | ~85% | Better than pristine membrane | [34] |
| Polyamide NF membranes modified with PVA | - | 3000 ppm MgSO4; 6 bar; 25 °C | 45.9 | 95.9 | Improved by adding PVA | [35] |
| Polyamide NF membranes modified with chitosan | - | 1000 ppm Na2SO4; 3 bar; 25 °C | 59.6 | 98% | Improved by adding chitosan | [36] |
| PAA–PAH NF membrane | ~70° | 1000 ppm MgSO4; 20 bar; 25 °C | 120 | >98% | Excellent fouling resistant by adding PAA–PAH | This Study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baig, U.; Waheed, A.; Salih, H.A.; Matin, A.; Alshami, A.; Aljundi, I.H. Facile Modification of NF Membrane by Multi-Layer Deposition of Polyelectrolytes for Enhanced Fouling Resistance. Polymers 2021, 13, 3728. https://doi.org/10.3390/polym13213728
Baig U, Waheed A, Salih HA, Matin A, Alshami A, Aljundi IH. Facile Modification of NF Membrane by Multi-Layer Deposition of Polyelectrolytes for Enhanced Fouling Resistance. Polymers. 2021; 13(21):3728. https://doi.org/10.3390/polym13213728
Chicago/Turabian StyleBaig, Umair, Abdul Waheed, Hassan A. Salih, Asif Matin, Ali Alshami, and Isam H. Aljundi. 2021. "Facile Modification of NF Membrane by Multi-Layer Deposition of Polyelectrolytes for Enhanced Fouling Resistance" Polymers 13, no. 21: 3728. https://doi.org/10.3390/polym13213728