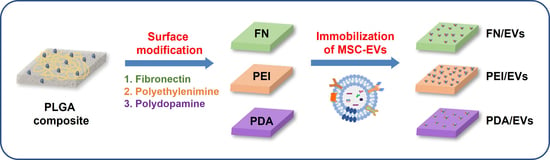
Comparison of Surface Functionalization of PLGA Composite to Immobilize Extracellular Vesicles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. Fabrication of PLGA Composite Films
2.4. Characterization of PLGA Composite Films
2.5. Isolation and Characterization of MSC-Derived EVs
2.6. Western Blot Analysis
2.7. Wound Healing Assay
2.8. Leukocyte Adhesion Assay
2.9. Tube Formation Assay
2.10. RNA Extraction and Quantitative Real-Time PCR
2.11. Statistical Analysis
3. Results and Discussion
3.1. Characterization of PLGA Composite Films
3.2. Characterization of MSC-Derived EVs
3.3. Immobilization of EVs on the Surface-Modified Composite Films
3.4. Effect of Released EVs from Composite Films on EC Function
3.5. Effect of EVs Released from Composite Films on Inflammation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nabel, E.G. Cardiovascular disease. N. Engl. J. Med. 2003, 349, 60–72. [Google Scholar] [CrossRef]
- Libby, P. Inflammation and cardiovascular disease mechanisms. Am. J. Clin. Nutr. 2006, 83, 456S–460S. [Google Scholar] [CrossRef] [Green Version]
- Berg, A.H.; Scherer, P.E. Adipose tissue, inflammation, and cardiovascular disease. Circ. Res. 2005, 96, 939–949. [Google Scholar] [CrossRef] [Green Version]
- Quyyumi, A.A. Endothelial function in health and disease: New insights into the genesis of cardiovascular disease. Am. J. Med. 1998, 105, 32S–39S. [Google Scholar] [CrossRef]
- Gimbrone, M.A., Jr.; García-Cardeña, G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ. Res. 2016, 118, 620–636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chistiakov, D.A.; Orekhov, A.N.; Bobryshev, Y.V. Endothelial Barrier and Its Abnormalities in Cardiovascular Disease. Front. Physiol. 2015, 6, 365. [Google Scholar] [CrossRef] [PubMed]
- Collins, T.; Read, M.A.; Neish, A.S.; Whitley, M.Z.; Thanos, D.; Maniatis, T. Transcriptional regulation of endothelial cell adhesion molecules: NF-kappa B and cytokine-inducible enhancers. FASEB J. 1995, 9, 899–909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pu, F.R.; Williams, R.L.; Markkula, T.K.; Hunt, J.A. Expression of leukocyte-endothelial cell adhesion molecules on monocyte adhesion to human endothelial cells on plasma treated PET and PTFE in vitro. Biomaterials 2002, 23, 4705–4718. [Google Scholar] [CrossRef]
- Haraldsen, G.; Kvale, D.; Lien, B.; Farstad, I.N.; Brandtzaeg, P. Cytokine-regulated expression of E-selectin, intercellular adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecule-1 (VCAM-1) in human microvascular endothelial cells. J. Immunol. 1996, 156, 2558–2565. [Google Scholar]
- Huo, Y.; Ley, K. Adhesion molecules and atherogenesis. Acta Physiol. Scand. 2001, 173, 35–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenwood, J.; Heasman, S.J.; Alvarez, J.I.; Prat, A.; Lyck, R.; Engelhardt, B. Review: Leucocyte-endothelial cell crosstalk at the blood-brain barrier: A prerequisite for successful immune cell entry to the brain. Neuropathol. Appl. Neurobiol. 2011, 37, 24–39. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.B.; Moncivais, K.; Caplan, A.I. Mesenchymal stem cells: Environmentally responsive therapeutics for regenerative medicine. Exp. Mol. Med. 2013, 45, e54. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.J.; Kwon, J.; Current, L.; Yoon, K.; Zalal, R.; Hu, X.; Xue, P.; Ko, C.C. Osteogenic potential of mesenchymal stem cells from rat mandible to regenerate critical sized calvarial defect. J. Tissue Eng. 2019, 10, 2041731419830427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, H.; Kyu-Cheol, N.; Wang, Z.; Cui, Y.; Liu, H.; Liu, H.; Feng, Y.; Zhao, Y.; Lin, Q.; Li, Z. Regulation of inflammatory microenvironment using a self-healing hydrogel loaded with BM-MSCs for advanced wound healing in rat diabetic foot ulcers. J. Tissue Eng. 2020, 11, 2041731420947242. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Baino, F.; Mauro, J.C.; Kumar, V.; Pickrell, G.; Sriranganathan, N.; Waldrop, S.G. Biomaterials for Cell Encapsulation: Progress Toward Clinical Applications. In Clinical Applications of Biomaterials; Springer: Berlin/Heidelberg, Germany, 2017; pp. 425–458. [Google Scholar]
- Lai, R.C.; Arslan, F.; Lee, M.M.; Sze, N.S.; Choo, A.; Chen, T.S.; Salto-Tellez, M.; Timmers, L.; Lee, C.N.; El Oakley, R.M.; et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010, 4, 214–222. [Google Scholar] [CrossRef] [Green Version]
- Keshtkar, S.; Azarpira, N.; Ghahremani, M.H. Mesenchymal stem cell-derived extracellular vesicles: Novel frontiers in regenerative medicine. Stem Cell Res. Ther. 2018, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Zhou, J.; Liang, C.; Liu, B.; Pan, X.; Zhang, Y.; Wang, Y.; Yan, B.; Xie, W.; Liu, F.; et al. Human umbilical cord mesenchymal stem cell derived exosomes encapsulated in functional peptide hydrogels promote cardiac repair. Biomater. Sci. 2019, 7, 2920–2933. [Google Scholar] [CrossRef]
- Cha, H.; Hong, S.; Park, J.H.; Park, H.H. Stem Cell-Derived Exosomes and Nanovesicles: Promotion of Cell Proliferation, Migration, and Anti-Senescence for Treatment of Wound Damage and Skin Ageing. Pharmaceutics 2020, 12, 1135. [Google Scholar] [CrossRef]
- Rostom, D.M.; Attia, N.; Khalifa, H.M.; Abou Nazel, M.W.; El Sabaawy, E.A. The Therapeutic Potential of Extracellular Vesicles Versus Mesenchymal Stem Cells in Liver Damage. Tissue Eng. Regen. Med. 2020, 17, 537–552. [Google Scholar] [CrossRef]
- Hu, S.; Li, Z.; Shen, D.; Zhu, D.; Huang, K.; Su, T.; Dinh, P.U.; Cores, J.; Cheng, K. Exosome-eluting stents for vascular healing after ischaemic injury. Nat. Biomed. Eng. 2021, 5, 1174–1188. [Google Scholar] [CrossRef]
- Yan, W.; Li, T.; Yin, T.; Hou, Z.; Qu, K.; Wang, N.; Durkan, C.; Dong, L.; Qiu, J.; Gregersen, H.; et al. M2 macrophage-derived exosomes promote the c-KIT phenotype of vascular smooth muscle cells during vascular tissue repair after intravascular stent implantation. Theranostics 2020, 10, 10712–10728. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Wang, F.; Zhang, J.; Cui, Y.; Pan, L.; Zhang, W.; Wen, J.; Liu, P. Exosomes of Endothelial Progenitor Cells Inhibit Neointima Formation After Carotid Artery Injury. J. Surg. Res. 2018, 232, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.I.; Rhim, W.K.; Kang, E.Y.; Choi, B.; Kim, J.H.; Han, D.K. A Multilayer Functionalized Drug-Eluting Balloon for Treatment of Coronary Artery Disease. Pharmaceutics 2021, 13, 614. [Google Scholar] [CrossRef]
- Rahdar, A.; Taboada, P.; Hajinezhad, M.R.; Barani, M.; Beyzaei, H. Effect of tocopherol on the properties of Pluronic F127 microemulsions: Physico-chemical characterization and in vivo toxicity. J. Mol. Liq. 2019, 277, 624–630. [Google Scholar] [CrossRef]
- Purushothaman, A.; Bandari, S.K.; Liu, J.; Mobley, J.A.; Brown, E.E.; Sanderson, R.D. Fibronectin on the Surface of Myeloma Cell-derived Exosomes Mediates Exosome-Cell Interactions. J. Biol. Chem. 2016, 291, 1652–1663. [Google Scholar] [CrossRef] [Green Version]
- Xie, H.; Wang, Z.; Zhang, L.; Lei, Q.; Zhao, A.; Wang, H.; Li, Q.; Cao, Y.; Zhang, W.J.; Chen, Z. Extracellular Vesicle-functionalized Decalcified Bone Matrix Scaffolds with Enhanced Pro-angiogenic and Pro-bone Regeneration Activities. Sci. Rep. 2017, 7, 45622. [Google Scholar] [CrossRef]
- Diomede, F.; Gugliandolo, A.; Cardelli, P.; Merciaro, I.; Ettorre, V.; Traini, T.; Bedini, R.; Scionti, D.; Bramanti, A.; Nanci, A.; et al. Three-dimensional printed PLA scaffold and human gingival stem cell-derived extracellular vesicles: A new tool for bone defect repair. Stem Cell Res. Ther. 2018, 9, 104. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Liu, Y.; Zhang, P.; Tang, Y.; Zhou, M.; Jiang, W.; Zhang, X.; Wu, G.; Zhou, Y. Tissue-Engineered Bone Immobilized with Human Adipose Stem Cells-Derived Exosomes Promotes Bone Regeneration. ACS Appl. Mater. Interfaces 2018, 10, 5240–5254. [Google Scholar] [CrossRef]
- Ko, K.W.; Park, S.Y.; Lee, E.H.; Yoo, Y.I.; Kim, D.S.; Kim, J.Y.; Kwon, T.G.; Han, D.K. Integrated Bioactive Scaffold with Polydeoxyribonucleotide and Stem-Cell-Derived Extracellular Vesicles for Kidney Regeneration. ACS Nano 2021, 15, 7575–7585. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.I.; Heo, Y.; Baek, S.W.; Kim, D.S.; Song, D.H.; Han, D.K. Multifunctional Biodegradable Vascular PLLA Scaffold with Improved X-ray Opacity, Anti-Inflammation, and Re-Endothelization. Polymers 2021, 13, 1979. [Google Scholar] [CrossRef] [PubMed]
- Ha, Y.M.; Kim, Y.N.; Jung, Y.C. Rapid and Local Self-Healing Ability of Polyurethane Nanocomposites Using Photothermal Polydopamine-Coated Graphene Oxide Triggered by Near-Infrared Laser. Polymers 2021, 13, 1274. [Google Scholar] [CrossRef]
- Jain, R.A. The manufacturing techniques of various drug loaded biodegradable poly(lactide-co-glycolide) (PLGA) devices. Biomaterials 2000, 21, 2475–2490. [Google Scholar] [CrossRef]
- Bedair, T.M.; Lee, C.K.; Kim, D.S.; Baek, S.W.; Bedair, H.M.; Joshi, H.P.; Choi, U.Y.; Park, K.H.; Park, W.; Han, I.; et al. Magnesium hydroxide-incorporated PLGA composite attenuates inflammation and promotes BMP2-induced bone formation in spinal fusion. J. Tissue Eng. 2020, 11, 2041731420967591. [Google Scholar] [CrossRef]
- Ko, K.W.; Choi, B.; Kang, E.Y.; Shin, S.W.; Baek, S.W.; Han, D.K. The antagonistic effect of magnesium hydroxide particles on vascular endothelial activation induced by acidic PLGA degradation products. Biomater. Sci. 2021, 9, 892–907. [Google Scholar] [CrossRef]
- Badylak, S.F. The extracellular matrix as a scaffold for tissue reconstruction. Semin. Cell Dev. Biol. 2002, 13, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Diomede, F.; D’Aurora, M.; Gugliandolo, A.; Merciaro, I.; Ettorre, V.; Bramanti, A.; Piattelli, A.; Gatta, V.; Mazzon, E.; Fontana, A.; et al. A novel role in skeletal segment regeneration of extracellular vesicles released from periodontal-ligament stem cells. Int. J. Nanomed. 2018, 13, 3805–3825. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Sample | XPS Atomic Composition (%) | Water Contact Angle (Degree) | ||
|---|---|---|---|---|
| C | O | N | ||
| PLGA | 73.46 | 25.18 | - | 89.3 ± 1.0 |
| PME | 73.59 | 25.61 | - | 73.7 ± 1.0 |
| PME/FN | 64.87 | 26.93 | 7.52 | 58.8 ± 1.0 |
| PME/PEI | 63.64 | 26.82 | 8.15 | 69.5 ± 4.2 |
| PME/PDA | 78.58 | 18.75 | 2.67 | 47.5 ± 1.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woo, J.; Ko, K.-W.; Cha, S.-G.; Heo, Y.; Han, D.K. Comparison of Surface Functionalization of PLGA Composite to Immobilize Extracellular Vesicles. Polymers 2021, 13, 3643. https://doi.org/10.3390/polym13213643
Woo J, Ko K-W, Cha S-G, Heo Y, Han DK. Comparison of Surface Functionalization of PLGA Composite to Immobilize Extracellular Vesicles. Polymers. 2021; 13(21):3643. https://doi.org/10.3390/polym13213643
Chicago/Turabian StyleWoo, Jiwon, Kyoung-Won Ko, Seung-Gyu Cha, Yun Heo, and Dong Keun Han. 2021. "Comparison of Surface Functionalization of PLGA Composite to Immobilize Extracellular Vesicles" Polymers 13, no. 21: 3643. https://doi.org/10.3390/polym13213643