An Engineered Specificity of Anti-Neoplastic Agent Loaded Magnetic Nanoparticles for the Treatment of Breast Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. ATR-FTIR Characterization
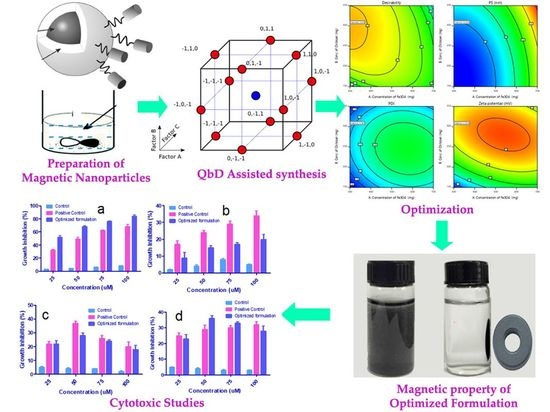
2.3. Preparation of the Magnetic Fe3O4 Nanoparticles
2.4. Synthesis of Fe3O4 NP@CS (Fe3O4@chitosan)
2.5. Preparation of Fe3O4@CS/Gemcitabine
2.6. Optimization of Fe3O4@CS MNP
2.7. Characterization
2.7.1. Particle Size and Distribution
2.7.2. Estimation of Surface Charge
2.7.3. Rationale of Experimental Design
2.8. Drug Loading and Surface Binding
2.9. Scanning Electron Microscopy (SEM)
2.10. Magnetization Measurement
2.11. In-Vitro Drug Release Profile
2.12. In Vitro Cytotoxicity Studies
2.12.1. Cell Culture
2.12.2. Cell Treatment
3. Results and Discussion
3.1. Fourier-Transform Infrared Radiation
3.2. Optimization of Fe3O4@CS MNP
+ 8.75 BC + 17.30 A² + 8.05 B² + 10.80 C²
− 0.0310 A² − 0.0435 B² + 0.0340 C²
+0.2500 BC − 3.40 A² − 8.15 B² − 4.90 C²
3.3. PS, PDI and Surface Charge
3.4. Surface Bindingand SEM
3.5. Measurement of Magnetization
3.6. In Vitro Drug Release
3.7. Cytotoxicity Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ren, J.-S.; Masuyer, E.; Ferlay, J. Global estimates of cancer prevalence for 27 sites in the adult population in 2008. Int. J. Cancer 2013, 132, 1133–1145. [Google Scholar] [CrossRef]
- Druker, B.J.; Talpaz, M.; Resta, D.J.; Peng, B.; Buchdunger, E.; Ford, J.M.; Lydon, N.B.; Kantarjian, H.; Capdeville, R.; Ohno-Jones, S.; et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N. Engl. J. Med. 2001, 344, 1031–1037. [Google Scholar] [CrossRef] [Green Version]
- Murphy, C.G.; Morris, P.G. Recent advances in novel targeted therapies for HER2-positive breast cancer. Anti-Cancer Drugs 2012, 23, 765–776. [Google Scholar] [CrossRef]
- Dürr, S.; Janko, C.; Lyer, S.; Tripal, P.; Schwarz, M.; Zaloga, J.; Tietze, R.; Alexiou, C. Magnetic nanoparticles for cancer therapy. Nanotechnol. Rev. 2013, 2, 395–409. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, M. Nano cancer therapy strategies. J. Cancer Res. Ther. 2012, 8, 19–22. [Google Scholar] [CrossRef]
- Durr, S.; Tietze, R.; Lyer, S.; Alexiou, C. Nanomedizin in der HNO-Heilkunde—Ein Ausblick. Laryngo-Rhino-Otologie 2012, 91, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.-M.; Jeong, H.-J. Current status and future direction of nanomedicine: Focus on advanced biological and medical applications. Nucl. Med. Mol. Imaging 2016, 51, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Ribeiro, A.M. Biomimetic nanoparticles: Preparation, characterization and biomedical applications. Int. J. Nanomed. 2010, 5, 249–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thanh, N.T.; Green, L.A. Functionalisation of nanoparticles for biomedical applications. Nano Today 2010, 5, 213–230. [Google Scholar] [CrossRef]
- Naud, C.; Thébault, C.; Carrière, M.; Hou, Y.; Morel, R.; Berger, F.; Diény, B.; Joisten, H. Cancer treatment by magneto-mechanical effect of particles, a review. Nanoscale Adv. 2020, 2, 3632–3655. [Google Scholar] [CrossRef]
- Awad, N.S.; Paul, V.; AlSawaftah, N.M.; ter Haar, G.; Allen, T.M.; Pitt, W.G.; Husseini, G.A. Ultrasound-responsive nanocarriers in cancer treatment: A review. ACS Pharmacol. Transl. Sci. 2021, 4, 589–612. [Google Scholar] [CrossRef]
- Chen, M.; Wu, J.; Ning, P.; Wang, J.; Ma, Z.; Huang, L.; Plaza, G.R.; Shen, Y.; Xu, C.; Han, Y.; et al. Remote control of mechanical forces via. mitochondrial-targeted magnetic nanospinners for efficient cancer treatment. Small 2020, 16, e1905424. [Google Scholar] [CrossRef]
- Sriraman, S.K.; Aryasomayajula, B.; Torchilin, V.P. Barriers to drug delivery in solid tumors. Tissue Barriers 2014, 2, e29528. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Wang, X.; Tang, X.; Hong, R.; Wang, Y.; Feng, W. Preparation and characterization of carbonyl iron/strontium hexaferrite magnetorheological fluids. Particuology 2015, 22, 134–144. [Google Scholar] [CrossRef]
- Singh, A.; Sahoo, S.K. Magnetic nanoparticles: A novel platform for cancer theranostics. Drug Discov. Today 2014, 19, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.S.; Tonga, G.Y.; Solfiell, D.; Rotello, V.M. Inorganic nanosystems for therapeutic delivery: Status and prospects. Adv. Drug Deliv. Rev. 2013, 65, 93–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angelakeris, M. Magnetic nanoparticles: A multifunctional vehicle for modern theranostics. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 1642–1651. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, G.R.; Ruiz-Morón, L.F.; Durán, J.D.G.; Delgado, A. Dynamic and wear study of an extremely bidisperse magnetorheological fluid. Smart Mater. Struct. 2015, 24, 127001. [Google Scholar] [CrossRef] [Green Version]
- Estelrich, J.; Escribano, E.; Queralt, J.; Busquets, M.A. Iron oxide nanoparticles for magnetically-guided and magnetically-responsive drug delivery. Int. J. Mol. Sci. 2015, 16, 8070–8101. [Google Scholar] [CrossRef] [Green Version]
- Klostergaard, J.; Seeney, C.E. Magnetic nanovectors for drug delivery. Nanomed. Nanotechnol. Biol. Med. 2012, 8, S37–S50. [Google Scholar] [CrossRef]
- Yao, L.; Xu, S. Detection of magnetic nanomaterials in molecular imaging and diagnosis applications. Nanotechnol. Rev. 2014, 3, 247–268. [Google Scholar] [CrossRef]
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [Green Version]
- Bernkop-Schnürch, A.; Dünnhaupt, S. Chitosan-based drug delivery systems. Eur. J. Pharm. Biopharm. 2012, 81, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Ghezeli, Z.K.; Hekmati, M.; Veisi, H. Synthesis of Imatinib-loaded chitosan-modified magnetic nanoparticles as an anti-cancer agent for pH responsive targeted drug delivery. Appl. Organomet. Chem. 2019, 33, e4833. [Google Scholar] [CrossRef]
- Sreeharsha, N.; Rajpoot, K.; Tekade, M.; Kalyane, D.; Nair, A.B.; Venugopala, K.N.; Tekade, R.K. Development of metronidazole loaded chitosan nanoparticles using QbD approach—A novel and potential antibacterial formulation. Pharmaceutics 2020, 12, 920. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; Nikles, D.E.; Brazel, C.S. Synthesis and characterization of multifunctional chitosan-MnFe2O4 nanoparticles for magnetic hyperthermia and drug delivery. Materials 2010, 3, 4051–4065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilczewska, A.Z.; Niemirowicz, K.; Markiewicz, K.H.; Car, H. Nanoparticles as drug delivery systems. Pharmacol. Rep. 2012, 64, 1020–1037. [Google Scholar] [CrossRef]
- Reddy, L.; Couvreur, L.H.R.A.P. Novel approaches to deliver gemcitabine to cancers. Curr. Pharm. Des. 2008, 14, 1124–1137. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.B.; Shah, J.; Al-Dhubiab, B.E.; Patel, S.S.; Morsy, M.A.; Patel, V.; Chavda, V.; Jacob, S.; Sreeharsha, N.; Shinu, P.; et al. Development of asialoglycoprotein receptor-targeted nanoparticles for selective delivery of gemcitabine to hepatocellular carcinoma. Molecules 2019, 24, 4566. [Google Scholar] [CrossRef] [Green Version]
- Toschi, L.; Finocchiaro, G.; Bartolini, S.; Gioia, V.; Cappuzzo, F. Role of gemcitabine in cancer therapy. Fut. Oncol. 2005, 1, 7–17. [Google Scholar] [CrossRef]
- Dasanu, C.A. Gemcitabine: Vascular toxicity and prothrombotic potential. Expert Opin. Drug Saf. 2008, 7, 703–716. [Google Scholar] [CrossRef] [PubMed]
- Arya, G.; Vandana, M.; Acharya, S.; Sahoo, S.K. Enhanced antiproliferative activity of herceptin (HER2)-conjugated gemcitabine-loaded chitosan nanoparticle in pancreatic cancer therapy. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 859–870. [Google Scholar] [CrossRef] [PubMed]
- Atyabi, F.; Dinarvand, R. Chitosan-pluronic nanoparticles as oral delivery of anticancer gemcitabine: Preparation and In Vitro study. Int. J. Nanomed. 2012, 7, 1851–1863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garg, N.K.; Dwivedi, P.; Campbell, C.; Tyagi, R.K. Site specific/targeted delivery of gemcitabine through anisamide anchored chitosan/poly ethylene glycol nanoparticles: An improved understanding of lung cancer therapeutic intervention. Eur. J. Pharm. Sci. 2012, 47, 1006–1014. [Google Scholar] [CrossRef] [PubMed]
- Arias, J.L.; Reddy, L.H.; Couvreur, P.; Patrick, C. Superior preclinical efficacy of gemcitabine developed as chitosan nanoparticulate system. Biomacromolecules 2011, 12, 97–104. [Google Scholar] [CrossRef]
- Parsian, M.; Mutlu, P.; Yalcin, S.; Gunduz, U. Characterization of gemcitabine loaded polyhydroxybutyrate coated magnetic nanoparticles for targeted drug delivery. Anti-Cancer Agents Med. Chem. 2020, 20, 1233–1240. [Google Scholar] [CrossRef]
- Rodrigues, S.; Dionísio, M.; López, C.R.; Grenha, A. Biocompatibility of chitosan carriers with application in drug delivery. J. Funct. Biomater. 2012, 3, 615–641. [Google Scholar] [CrossRef] [Green Version]
- Naveen, N.R.; Gopinath, C.; Kurakula, M. Okra-thioglycolic acid conjugate—synthesis, characterization, and evaluation as a mucoadhesive polymer. Processes 2020, 8, 316. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, O.A.A.; Kurakula, M.; Banjar, Z.M.; Afouna, M.I.; Zidan, A.S. Quality by design coupled with near infrared in formulation of transdermal glimepiride liposomal films. J. Pharm. Sci. 2015, 104, 2062–2075. [Google Scholar] [CrossRef]
- Kurakula, M.; Naveen, N.R. In Situ gel loaded with chitosan-coated simvastatin nanoparticles: Promising delivery for effective anti-proliferative activity against tongue carcinoma. Mar. Drugs 2020, 18, 201. [Google Scholar] [CrossRef] [Green Version]
- Naveen, N.R.; Gopinath, C.; Rao, D.S. Design expert supported mathematical optimization of repaglinide gastroretentive floating tablets: In Vitro and In Vivo evaluation. Futur. J. Pharm. Sci. 2017, 3, 140–147. [Google Scholar] [CrossRef]
- Morsy, M.A.; Abdel-Latif, R.G.; Nair, A.B.; Venugopala, K.N.; Ahmed, A.F.; Elsewedy, H.S.; Shehata, T.M. Preparation and evaluation of atorvastatin-loaded nanoemulgel on wound-healing efficacy. Pharmaceutics 2019, 11, 609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nair, A.; Shah, J.; Al-Dhubiab, B.; Jacob, S.; Patel, S.; Venugopala, K.; Morsy, M.; Gupta, S.; Attimarad, M.; Sreeharsha, N.; et al. Clarithromycin solid lipid nanoparticles for topical ocular therapy: Optimization, evaluation and In Vivo studies. Pharmaceutics 2021, 13, 523. [Google Scholar] [CrossRef]
- Adetunji, A.I.; Olaniran, A.O. Statistical modelling and optimization of protease production by an autochthonous Bacillus aryabhattai Ab15-ES: A response surface methodology approach. Biocatal. Agric. Biotechnol. 2020, 24, 101528. [Google Scholar] [CrossRef]
- Parsian, M.; Unsoy, G.; Mutlu, P.; Yalcin, S.; Tezcaner, A.; Gunduz, U. Loading of gemcitabine on chitosan magnetic nanoparticles increases the anti-cancer efficacy of the drug. Eur. J. Pharmacol. 2016, 784, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.; Nair, A.B.; Aldhubiab, B.E. Preparation and evaluation of niosome gel containing acyclovir for enhanced dermal deposition. J. Liposome Res. 2017, 27, 283–292. [Google Scholar] [CrossRef]
- Morsy, M.A.; Nair, A.B. Prevention of rat liver fibrosis by selective targeting of hepatic stellate cells using hesperidin carriers. Int. J. Pharm. 2018, 552, 241–250. [Google Scholar] [CrossRef]
- Akrawi, S.H.; Gorain, B.; Nair, A.B.; Choudhury, H.; Pandey, M.; Shah, J.N.; Venugopala, K.N. Development and optimization of naringenin-loaded chitosan-coated nanoemulsion for topical therapy in wound healing. Pharmaceutics 2020, 12, 893. [Google Scholar] [CrossRef]
- Sreeharsha, N.; Hiremath, J.G.; Kumar, P.R.; Meravanige, G.; Khan, S.; Karnati, R.K.; Attimarad, M.; Al-Dhubiab, B.; Nair, A.B.; Venugopala, K.N. Doxorubicin hydrochloride loaded polyanhydride nanoformulations and cytotoxicity. Indian J. Pharm. Educ. Res. 2021, 55, 117–125. [Google Scholar] [CrossRef]
- Nair, A.; Al-Dhubiab, B.E.; Shah, J.; Attimarad, M. Poly (lactic acid-co-glycolic acid) nanospheres improved the oral delivery of candesartan cilexetil. Indian J. Pharm. Educ. Res. 2017, 51, 571–579. [Google Scholar] [CrossRef] [Green Version]
- Sarathchandiran, I.; Koumaravelou, K.; Selvasudha, N. Interaction pattern and In Vitro, In Vivo release behavior of simvastatin-loaded chitosan nanoformulation. Drug Dev. Ind. Pharm. 2019, 45, 1725–1739. [Google Scholar] [CrossRef]
- Hosny, K.M.; Aldawsari, H.M.; Bahmdan, R.H.; Sindi, A.M.; Kurakula, M.; Alrobaian, M.M.; Aldryhim, A.Y.; Alkhalidi, H.M.; Bahmdan, H.H.; Khallaf, R.A.; et al. Preparation, optimization, and evaluation of hyaluronic acid-based hydrogel loaded with miconazole self-nanoemulsion for the treatment of oral thrush. AAPS PharmSciTech 2019, 20, 297. [Google Scholar] [CrossRef] [PubMed]
- Kurakula, M.; Naveen, N.R.; Patel, B.; Manne, R.; Patel, D. Preparation, optimization and evaluation of chitosan-based avanafil nanocomplex utilizing antioxidants for enhanced neuroprotective effect on PC12 cells. Gels 2021, 7, 96. [Google Scholar] [CrossRef] [PubMed]
- Naveen, N.R.; Kurakula, M.; Gowthami, B. Process optimization by response surface methodology for preparation and evaluation of methotrexate loaded chitosan nanoparticles. Mater. Today Proc. 2020, 33, 2716–2724. [Google Scholar] [CrossRef]
- Shah, H.; Nair, A.B.; Shah, J.; Jacob, S.; Bharadia, P.; Haroun, M. Proniosomal vesicles as an effective strategy to optimize naproxen transdermal delivery. J. Drug Deliv. Sci. Technol. 2021, 63, 102479. [Google Scholar] [CrossRef]
- Shah, J.; Nair, A.B.; Jacob, S.; Patel, R.K.; Shah, H.; Shehata, T.M.; Morsy, M.A. Nanoemulsion based vehicle for effective ocular delivery of moxifloxacin using experimental design and pharmacokinetic study in rabbits. Pharmaceutics 2019, 11, 230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gopinath, D.; Ravi, D.; Rao, B.; Apte, S.; Renuka, D.; Rambhau, D. Ascorbyl palmitate vesicles (Aspasomes): Formation, characterization and applications. Int. J. Pharm. 2004, 271, 95–113. [Google Scholar] [CrossRef]
- Blazek-Welsh, A.I.; Rhodes, D.G. Maltodextrin-based proniosomes. AAPS PharmSci 2001, 3, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Nair, A.; Gupta, R.; Vasanti, S. In Vitro controlled release of alfuzosin hydrochloride using HPMC-based matrix tablets and its comparison with marketed product. Pharm. Dev. Technol. 2007, 12, 621–625. [Google Scholar] [CrossRef]










| Factors/Independent Variables | Levels | Responses/Dependent Variables | Constraints | ||
|---|---|---|---|---|---|
| −1 | 0 | +1 | |||
| Concentration of Fe3O4 (g)-X1 | 300 | 500 | 700 | Particle size (nm) | Minimum |
| Conc. of Chitosan (g)-X2 | 150 | 250 | 350 | Polydispersity index | Minimum |
| Sonication time (h)-X3 | 1.5 | 2 | 2.5 | Zeta potential (mV) | Maximum |
| Run | A:Conc. of Fe3O4 (g) | B:Conc. of Chitosan (g) | C:Sonication Time (h) | Particle Size (nm) | Polydispersity Index | Zeta Potential (mV) |
|---|---|---|---|---|---|---|
| 1 | 500 | 250 | 2 | 69 | 0.36 | 36 |
| 2 | 500 | 350 | 1 | 105 | 0.42 | 27 |
| 3 | 500 | 250 | 2 | 68 | 0.36 | 38 |
| 4 | 700 | 250 | 1 | 142 | 0.47 | 29 |
| 5 | 500 | 150 | 1 | 96 | 0.43 | 18 |
| 6 | 300 | 250 | 3 | 54 | 0.29 | 25 |
| 7 | 500 | 250 | 2 | 71 | 0.38 | 33 |
| 8 | 700 | 250 | 3 | 119 | 0.33 | 29 |
| 9 | 700 | 150 | 2 | 124 | 0.32 | 24 |
| 10 | 500 | 350 | 3 | 98 | 0.29 | 28 |
| 11 | 700 | 350 | 2 | 145 | 0.32 | 30 |
| 12 | 500 | 250 | 2 | 70 | 0.39 | 35 |
| 13 | 300 | 350 | 2 | 65 | 0.29 | 31 |
| 14 | 500 | 150 | 3 | 54 | 0.31 | 18 |
| 15 | 500 | 250 | 2 | 69 | 0.37 | 37 |
| 16 | 300 | 250 | 1 | 75 | 0.41 | 27 |
| 17 | 300 | 150 | 2 | 45 | 0.26 | 12 |
| Particle Size | Polydispersity Index | Zeta Potential | |
|---|---|---|---|
| Std. Dev. | 4.10 | 0.0128 | 1.83 |
| Mean | 86.41 | 0.3529 | 28.06 |
| C.V. % | 4.74 | 3.64 | 6.54 |
| Sequential p-value | <0.0001 | 0.0002 | <0.0001 |
| Lack of Fit p-value | 0.0636 | 0.5034 | 0.5600 |
| R² | 0.9826 | 0.9790 | 0.9712 |
| Adjusted R² | 0.9418 | 0.9520 | 0.9341 |
| Predicted R² | 0.8830 | 0.8424 | 0.8003 |
| Adeq. Precision | 31.552 | 20.4276 | 15.9408 |
| Intercept | A | B | C | AB | AC | BC | A² | B² | C² | |
|---|---|---|---|---|---|---|---|---|---|---|
| Particle size | 36.375 | 11.75 | −11.625 | 0.25 | −0.5 | 8.75 | 17.3 | 8.05 | 10.8 | 36.375 |
| p-values | <0.0001 | <0.0001 | <0.0001 | 0.9063 | 0.8141 | 0.0037 | <0.0001 | 0.0050 | 0.0010 | <0.0001 |
| Polydispersity index | 0.02375 | −2.95384 × 10 −17 | −0.06375 | −0.0075 | −0.005 | −0.0025 | −0.031 | −0.0435 | 0.034 | 0.02375 |
| p-values | 0.0012 | 1.0000 | <0.0001 | 0.2811 | 0.4618 | 0.7087 | 0.0017 | 0.0002 | 0.0010 | 0.0012 |
| Zeta potential | 2.125 | 5.5 | −0.125 | −3.25 | 0.5 | 0.25 | −3.4 | −8.15 | −4.9 | 2.125 |
| p-values | 0.0135 | <0.0001 | 0.8526 | 0.0094 | 0.6025 | 0.7930 | 0.0067 | <0.0001 | 0.0009 | 0.0135 |
| Solution 1 of 12 Response | Predicted Median | 95% CI Low for Mean | 95% CI High for Mean | 95% TI Low for 99% Pop | 95% TI High for 99% Pop |
|---|---|---|---|---|---|
| Particle size | 56.5295 | 50.1251 | 62.9338 | 32.4364 | 80.6225 |
| Polydispersity index | 0.2941 | 0.274017 | 0.314184 | 0.218547 | 0.369654 |
| Zeta potential | 31.8148 | 28.9471 | 34.6826 | 21.0263 | 42.6033 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nair, A.B.; Telsang, M.; Osmani, R.A. An Engineered Specificity of Anti-Neoplastic Agent Loaded Magnetic Nanoparticles for the Treatment of Breast Cancer. Polymers 2021, 13, 3623. https://doi.org/10.3390/polym13213623
Nair AB, Telsang M, Osmani RA. An Engineered Specificity of Anti-Neoplastic Agent Loaded Magnetic Nanoparticles for the Treatment of Breast Cancer. Polymers. 2021; 13(21):3623. https://doi.org/10.3390/polym13213623
Chicago/Turabian StyleNair, Anroop B., Mallikarjun Telsang, and Riyaz Ali Osmani. 2021. "An Engineered Specificity of Anti-Neoplastic Agent Loaded Magnetic Nanoparticles for the Treatment of Breast Cancer" Polymers 13, no. 21: 3623. https://doi.org/10.3390/polym13213623