Regenerated Cellulose Products for Agricultural and Their Potential: A Review
Abstract
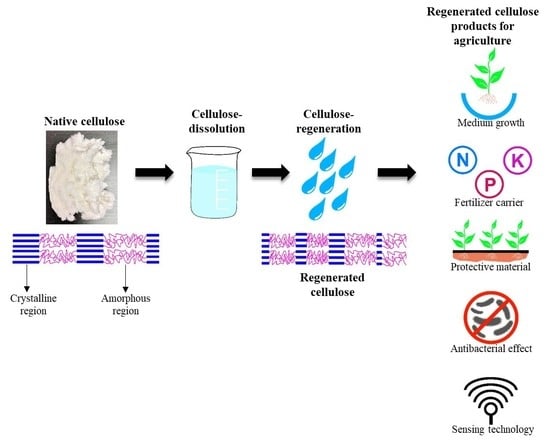
:1. Introduction
2. Regenerated Cellulose Products
2.1. Hydrogel, Aerogel, Cryogel, and Xerogel
| Type of Product | Method of Production | Properties | Appearance |
|---|---|---|---|
| Aerogel | First developed by Kristler in the 1930s performed by a supercritical drying to remove liquid from hydrogel [69] | Low density, large surface area, excellent mechanical properties, and high porosity. Sometimes, brittle aerogel is produced due to the drying technique applied. Commonly as an adsorption material. | White, opaque, lightweight |
| Cryogel | Hydrogel is frozen and lyophilizes at very low temperature to remove liquid | Lightweight and low density. Compact structure and large porosity depend on freezing temperature and ice crystals formation during freezing. | White, opaque, lightweight |
| Xerogel | Low-evaporative or vacuum drying of hydrogel and subsequently forming a thin porous film with extreme shrinkage [70]. | Mimicking aerogel but improved in brittleness characteristic that is commonly generated from aerogel [68]. Dense, compact, and very low porosity. | Yellowish, shrunk, translucent |
2.2. Fibers
2.3. Membrane and Thin Film
3. The Potential Applications of Regenerated Cellulose Products for Agriculture
3.1. Excellent Water Conserver for Plantation Media
3.2. Long-Shelf Life Plantation Media
3.3. Nutrient Reservoir
3.4. Protective Materials
3.5. Biosensors
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Shennan, C.; Krupnik, T.J.; Baird, G.; Cohen, H.; Forbush, K.; Lovell, R.; Olimpi, E.M. Organic and Conventional Agriculture: A Useful Framing? Annu. Rev. Environ. Res. 2017, 42, 317–346. [Google Scholar] [CrossRef]
- Kılıç, O.; Boz, I.; Eryılmaz, G.A. Comparison of conventional and good agricultural practices farms: A socio-economic and technical perspective. J. Clean. Prod. 2020, 258, 120666. [Google Scholar] [CrossRef]
- Tal, A. Making Conventional Agriculture Environmentally Friendly: Moving beyond the Glorification of Organic Agriculture and the Demonization of Conventional Agriculture. Sustainability 2018, 10, 1078. [Google Scholar] [CrossRef] [Green Version]
- Cueff, S.; Alletto, L.; Bourdat-Deschamps, M.; Benoit, P.; Pot, V. Water and pesticide transfers in undisturbed soil columns sampled from a Stagnic Luvisol and a Vermic Umbrisol both cultivated under conventional and conservation agriculture. Geoderma 2020, 377, 114590. [Google Scholar] [CrossRef]
- Alcamo, J. Water quality and its interlinkages with the Sustainable Development Goals. Curr. Opin. Environ. Sustain. 2019, 36, 126–140. [Google Scholar] [CrossRef]
- Wojtkowski, P. Agroecology; Springer: Cham, Switzerland, 2019; pp. 173–189. [Google Scholar] [CrossRef]
- Kumar, M. Agricultural-Based Interventions for Sustainable Food Security & Climate Change; AkiNik Publications: New Delhi, India, 2018. [Google Scholar]
- Maji, B. Introduction to Natural Polysaccharides; Elsevier Ltd.: Philadelphia, PA, USA, 2019; pp. 1–31. [Google Scholar]
- Rozo, G.; Bohorques, L.; Santamaría, J. Controlled release fertilizer encapsulated by a κ-carrageenan hydrogel. Polímeros 2019, 29, 1–12. [Google Scholar] [CrossRef]
- Kaco, H.; Zakaria, S.; Chia, C.H.; Zhang, L. Transparent and Printable Regenerated Kenaf Cellulose/PVA Film. Bioresources 2014, 9, 2167–2178. [Google Scholar] [CrossRef]
- Salleh, K.M.; Zakaria, S.; Sajab, M.S.; Gan, S.; Chia, C.H.; Jaafar, S.N.S.; Amran, U.A. Chemically crosslinked hydrogel and its driving force towards superabsorbent behaviour. Int. J. Biol. Macromol. 2018, 118, 1422–1430. [Google Scholar] [CrossRef]
- Singh, P.; Duarte, H.; Alves, L.; Antunes, F.; Le Moigne, N.; Dormanns, J.; Duchemin, B.; Staiger, M.P.; Medronho, B. From Cellulose Dissolution and Regeneration to Added Value Applications—Synergism Between Molecular Understanding and Material Development. In Cellulose-Fundamental Aspects and Current Trends; IntechOpen: London, UK, 2015; pp. 1–44. [Google Scholar] [CrossRef] [Green Version]
- Orzali, L.; Corsi, B.; Forni, C.; Riccioni, L. Chitosan in Agriculture: A New Challenge for Managing Plant Disease. In Biological Activities and Application of Marine Polysaccharides; InTech: London, UK, 2017. [Google Scholar]
- Llanes, L.; Dubessay, P.; Pierre, G.; Delattre, C.; Michaud, P. Biosourced Polysaccharide-Based Superabsorbents. Polysaccharides 2020, 1, 5. [Google Scholar] [CrossRef]
- Amran, U.A.; Zakaria, S.; Chia, C.H.; Roslan, R.; Jaafar, S.N.S.; Salleh, K.M. Polyols and rigid polyurethane foams derived from liquefied lignocellulosic and cellulosic biomass. Cellulose 2019, 26, 3231–3246. [Google Scholar] [CrossRef]
- Gan, S.; Zakaria, S.; Chia, C.H.; Kaco, H. Effect of graphene oxide on thermal stability of aerogel bio-nanocomposite from cellulose-based waste biomass. Cellulose 2018, 25, 5099–5112. [Google Scholar] [CrossRef]
- Wustenberg, T. Cellulose and Cellulose Derivatives in the Food Industry, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2012. [Google Scholar]
- Gupta, V.K.; Carrott, P.J.M.; Singh, R.; Chaudhary, M.; Kushwaha, S. Cellulose: A review as natural, modified and activated carbon adsorbent. Bioresour. Technol. 2016, 216, 1066–1076. [Google Scholar] [CrossRef]
- Baharin, K.W.; Zakaria, S.; Ellis, A.V.; Talip, N.; Kaco, H.; Gan, S.; Zailan, F.D.; Hashim, S.N.A.S. Factors affecting cellulose dissolution of oil palm empty fruit bunch and kenaf pulp in NaOH/urea solvent. St. Malays. 2018, 47, 377–386. [Google Scholar] [CrossRef]
- Poletto, M.; Ornaghi, J.H.L.; Zattera, A.J. Native Cellulose: Structure, Characterization and Thermal Properties. Material 2014, 7, 6105–6119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hidaka, H.; Kim, U.-J.; Wada, M. Synchrotron X-ray fiber diffraction study on the thermal expansion behavior of cellulose crystals in tension wood of Japanese poplar in the low-temperature region. Holzforschung 2010, 64, 167–171. [Google Scholar] [CrossRef]
- Qi, H.; Chang, C.; Zhang, L. Effects of temperature and molecular weight on dissolution of cellulose in NaOH/urea aqueous solution. Cellulose 2008, 15, 779–787. [Google Scholar] [CrossRef]
- Park, S.; Baker, J.O.; Himmel, M.E.; Parilla, P.A.; Johnson, D.K. Cellulose crystallinity index: Measurement techniques and their impact on interpreting cellulase performance. Biotechnol. Biofuels 2010, 3, 10. [Google Scholar] [CrossRef] [Green Version]
- Gan, S.Y.; Zakaria, S.; Chia, C.H.; Chen, R.S.; Ellis, A.; Kaco, H. Highly porous regenerated cellulose hydrogel and aerogel prepared from hydrothermal synthesized cellulose carbamate. PLoS ONE 2017, 12, e0173743. [Google Scholar] [CrossRef]
- Sayyed, A.; Deshmukh, N.A.; Pinjari, D.V. A critical review of manufacturing processes used in regenerated cellulosic fibres: Viscose, cellulose acetate, cuprammonium, LiCl/DMAc, ionic liquids, and NMMO based lyocell. Cellulose 2019, 26, 2913–2940. [Google Scholar] [CrossRef]
- Berga, L.; Bruce, I.; Nicol, T.W.J.; Holding, A.J.; Isobe, N.; Shimizu, S.; Walker, A.J.; Reid, J.E.S.J. Cellulose dissolution and regeneration using a non-aqueous, non-stoichiometric protic ionic liquid system. Cellulose 2020, 27, 9593–9603. [Google Scholar] [CrossRef]
- Egal, M.; Budtova, T.; Navard, P. Structure of Aqueous Solutions of Microcrystalline Cellulose/Sodium Hydroxide below 0 °C and the Limit of Cellulose Dissolution. Biomacromolecules 2007, 8, 2282–2287. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Chen, C.; Duan, C.; Hu, H.; Li, H.; Li, J.; Liu, Y.; Ma, X.; Stavik, J.; Ni, Y. Regenerated cellulose by the Lyocell process, a brief review of the process and properties. Bioresources 2018, 13, 4577–4592. [Google Scholar] [CrossRef]
- Kasprzak, D.; Krystkowiak, E.; Stępniak, I.; Galiński, M. Dissolution of cellulose in novel carboxylate-based ionic liquids and dimethyl sulfoxide mixed solvents. Eur. Polym. J. 2019, 113, 89–97. [Google Scholar] [CrossRef]
- Ma, Y.; Rissanen, M.; You, X.; Moriam, K.; Hummel, M.; Sixta, H. New method for determining the degree of fibrillation of regenerated cellulose fibres. Cellulose 2021, 28, 31–44. [Google Scholar] [CrossRef]
- Isobe, N.; Kimura, S.; Wada, M.; Kuga, S. Mechanism of cellulose gelation from aqueous alkali-urea solution. Carbohydr. Polym. 2012, 89, 1298–1300. [Google Scholar] [CrossRef]
- Liu, W.; Liu, S.; Liu, T.; Liu, T.; Zhang, J.; Liu, H. Eco-friendly post-consumer cotton waste recycling for regenerated cellulose fibers. Carbohydr. Polym. 2019, 206, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Chen, J.Y.; Jiang, W.; Lynch, V. Crystalline characteristics of cellulose fiber and film regenerated from ionic liquid solution. Carbohydr. Polym. 2015, 118, 150–155. [Google Scholar] [CrossRef]
- Elsayed, S.; Hellsten, S.; Guizani, C.; Witos, J.; Rissanen, M.; Rantamäki, A.H.; Varis, P.; Wiedmer, S.; Sixta, H. Recycling of Superbase-Based Ionic Liquid Solvents for the Production of Textile-Grade Regenerated Cellulose Fibers in the Lyocell Process. ACS Sustain. Chem. Eng. 2020, 8, 14217–14227. [Google Scholar] [CrossRef]
- Pang, J.; Wu, M.; Zhang, Q.; Tan, X.; Xu, F.; Zhang, X.; Sun, R. Comparison of physical properties of regenerated cellulose films fabricated with different cellulose feedstocks in ionic liquid. Carbohydr. Polym. 2015, 121, 71–78. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, X.; Hao, M.; Huang, C.; Xue, Z.; Mu, T. Preparation and characterization of regenerated cellulose from ionic liquid using different methods. Carbohydr. Polym. 2015, 117, 99–105. [Google Scholar] [CrossRef]
- Egal, M.; Budtova, T.; Navard, P. The dissolution of microcrystalline cellulose in sodium hydroxide-urea aqueous solutions. Cellulose 2007, 15, 361–370. [Google Scholar] [CrossRef]
- Tu, H.; Zhu, M.; Duan, B.; Zhang, L. Recent Progress in High-Strength and Robust Regenerated Cellulose Materials. Adv. Mater. 2021, 33, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Xiao, H.; Su, Y.; Wu, Y.; Cui, Y.; Li, M. Mechanical and physical properties of regenerated biomass composite films from lignocellulosic materials in ionic liquid. Bioresources 2019, 14, 2584–2595. [Google Scholar] [CrossRef]
- Santamala, H.; Livingston, R.; Sixta, H.; Hummel, M.; Skrifvars, M.; Saarela, O. Advantages of regenerated cellulose fibres as compared to flax fibres in the processability and mechanical performance of thermoset composites. Compos. Part A Appl. Sci. Manuf. 2016, 84, 377–385. [Google Scholar] [CrossRef]
- Zhou, Q.; Bao, Y.; Zhang, H.; Luan, Q.; Tang, H.; Li, X. Regenerated cellulose-based composite membranes as adsorbent for protein adsorption. Cellulose 2019, 27, 335–345. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, C.; Feng, X.; Wu, M.; Tang, Y.; Li, B. Effect of regeneration solvent on the characteristics of regenerated cellulose from lithium bromide trihydrate molten salt. Cellulose 2020, 27, 9243–9256. [Google Scholar] [CrossRef]
- Azmi, A.; Lau, K.S.; Chin, S.X.; Khiew, P.S.; Zakaria, S.; Chia, C.H. ZnO filled PVA cellulose nanifibril aerogel nanocomposite for catalytic decomposition of an organic dye in aqueous solution. Cellulose 2021, 28, 2241–2253. [Google Scholar] [CrossRef]
- Esfahani, M.R.; Taylor, A.; Serwinowski, N.; Parkerson, Z.; Confer, M.P.; Kammakakam, I.; Bara, J.E.; Esfahani, A.; Mahmoodi, S.N.; Koutahzadeh, N.; et al. Sustainable Novel Bamboo-Based Membranes for Water Treatment Fabricated by Regeneration of Bamboo Waste Fibers. ACS Sustain. Chem. Eng. 2020, 8, 4225–4235. [Google Scholar] [CrossRef]
- Adsul, M.; Soni, S.K.; Bhargava, S.K.; Bansal, V. Facile Approach for the Dispersion of Regenerated Cellulose in Aqueous System in the Form of Nanoparticles. Biomacromolecules 2012, 13, 2890–2895. [Google Scholar] [CrossRef]
- Song, B.; Liang, H.; Sun, R.; Peng, P.; Jiang, Y.; She, D. Hydrogel synthesis based on lignin/sodium alginate and application in agriculture. Int. J. Biol. Macromol. 2020, 144, 219–230. [Google Scholar] [CrossRef]
- Xing, L.; Hu, C.; Zhang, W.; Guan, L.; Gu, J. Transition of cellulose supramolecular structure during concentrated acid treatment and its implication for cellulose nanocrystal yield. Carbohydr. Polym. 2020, 229, 115539. [Google Scholar] [CrossRef]
- Tyshkunova, I.V.; Chukhchin, D.G.; Gofman, I.V.; Poshina, D.N.; Skorik, Y.A. Cellulose cryogels prepared by regeneration from phosphoric acid solutions. Cellulose 2021, 28, 1–15. [Google Scholar] [CrossRef]
- Li, N.; Bian, H.; Zhu, J.; Ciesielski, P.N.; Pan, X. Tailorable cellulose II nanocrystals (CNC II) prepared in mildly acidic lithium bromide trihydrate (MALBTH). Green Chem. 2021, 23, 2778–2791. [Google Scholar] [CrossRef]
- Pakutsah, K.; Aht-Ong, D. Eco-Friendly Preparation of Nanofibrillated Cellulose from Water Hyacinth Using NaOH/Urea Pretreatment. Mater. Sci. Forum 2020, 990, 225–230. [Google Scholar] [CrossRef]
- Bian, H.; Tu, P.; Chen, J.Y. Fabrication of all-cellulose nanocomposites from corn stalk. J. Sci. Food Agric. 2020, 100, 4390–4399. [Google Scholar] [CrossRef] [PubMed]
- Kabir, S.M.F.; Sikdar, P.P.; Haque, B.; Bhuiyan, M.A.R.; Ali, A.; Islam, M.N. Cellulose-based hydrogel materials: Chemistry, properties and their prospective applications. Prog. Biomater. 2018, 7, 153–174. [Google Scholar] [CrossRef] [Green Version]
- Maharjan, B.; Park, J.; Kaliannagounder, V.K.; Awasthi, G.P.; Joshi, M.K.; Park, C.H.; Kim, C.S. Regenerated cellulose nanofiber reinforced chitosan hydrogel scaffolds for bone tissue engineering. Carbohydr. Polym. 2021, 251, 117023. [Google Scholar] [CrossRef]
- Sasmal, P.K.; Patra, S. Effect in Growth of Corn Plant from Cellulose-Based Hydrogel Derived from Wheat Straw. J. Inst. Eng. Ser. E 2020, 1–6. [Google Scholar] [CrossRef]
- Hu, W.; Wang, Z.; Xiao, Y.; Zhang, S.; Wang, J. Advances in crosslinking strategies of biomedical hydrogels. Biomater. Sci. 2019, 7, 843–855. [Google Scholar] [CrossRef]
- Ranganathan, N.; Bensingh, R.J.; Kader, M.A.; Nayak, S.K. Synthesis and Properties of Hydrogels Prepared by Various Polymerization Reaction Systems; Springer International Publishing: Cham, Switzerland, 2019; pp. 487–511. [Google Scholar]
- Zhang, Q.; Wu, M.; Hu, X.; Lu, W.; Wang, M.; Li, T.; Zhao, Y. A Novel Double-Network, Self-Healing Hydrogel Based on Hydrogen Bonding and Hydrophobic Effect. Macromol. Chem. Phys. 2019, 221, 1900320. [Google Scholar] [CrossRef]
- Treesuppharat, W.; Rojanapanthu, P.; Siangsanoh, C.; Manuspiya, H.; Ummartyotin, S. Synthesis and characterization of bacterial cellulose and gelatin-based hydrogel composites for drug-delivery systems. Biotechnol. Rep. 2017, 15, 84–91. [Google Scholar] [CrossRef] [PubMed]
- De France, K.; D’Emilio, E.; Cranston, E.D.; Geiger, T.; Nyström, G. Dual physically and chemically crosslinked regenerated cellulose—Gelatin composite hydrogels towards art restoration. Carbohydr. Polym. 2020, 234, 115885. [Google Scholar] [CrossRef] [PubMed]
- Teng, J.; Yang, B.; Zhang, L.-Q.; Lin, S.-Q.; Xu, L.; Zhong, G.-J.; Tang, J.-H.; Li, Z.-M. Ultra-high mechanical properties of porous composites based on regenerated cellulose and cross-linked poly(ethylene glycol). Carbohydr. Polym. 2018, 179, 244–251. [Google Scholar] [CrossRef]
- Yang, B.; Hua, W.; Li, L.; Zhou, Z.; Xu, L.; Bian, F.; Ji, X.; Zhong, G.; Li, Z. Robust hydrogel of regenerated cellulose by chemical crosslinking coupled with polyacrylamide network. J. Appl. Polym. Sci. 2019, 136, 1–10. [Google Scholar] [CrossRef]
- Xia, Z.; Li, J.; Zhang, J.; Zhang, X.; Zheng, X.; Zhang, J. Processing and valorization of cellulose, lignin and lignocellulose using ionic liquids. J. Bioresour. Bioprod. 2020, 5, 79–95. [Google Scholar] [CrossRef]
- Seiler, E.; Koyama, K.; Iijima, T.; Saito, T.; Takeoka, Y.; Rikukawa, M.; Yoshizawa-Fujita, M. Simple and Fast One-Pot Cellulose Gel Preparation in Aqueous Pyrrolidinium Hydroxide Solution–Cellulose Solvent and Antibacterial Agent. Polymers 2021, 13, 1942. [Google Scholar] [CrossRef]
- Karadagli, I.; Schulz, B.; Schestakow, M.; Milow, B.; Gries, T.; Ratke, L. Production of porous cellulose aerogel fibers by an extrusion process. J. Supercrit. Fluids 2015, 106, 105–114. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, H.; Hou, D.; Tan, H.; Yang, M. Regenerated cellulose aerogel: Morphology control and the application as the template for functional cellulose nanoparticles. J. Appl. Polym. Sci. 2020, 137, 1–11. [Google Scholar] [CrossRef]
- Liu, S.; Tao, D.; Zhang, L. Cellulose scaffold: A green template for the controlling synthesis of magnetic inorganic nanoparticles. Powder Technol. 2012, 217, 502–509. [Google Scholar] [CrossRef]
- Ciolacu, D.; Rudaz, C.; Vasilescu, M.; Budtova, T. Physically and chemically cross-linked cellulose cryogels: Structure, properties and application for controlled release. Carbohydr. Polym. 2016, 151, 392–400. [Google Scholar] [CrossRef]
- Yamasaki, S.; Sakuma, W.; Yasui, H.; Daicho, K.; Saito, T.; Fujisawa, S.; Isogai, A.; Kanamori, K. Nanocellulose Xerogels with High Porosities and Large Specific Surface Areas. Front. Chem. 2019, 7, 316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, L.-Y.; Weng, Y.-X.; Wang, Y.-Z. Cellulose Aerogels: Synthesis, Applications, and Prospects. Polymers 2018, 10, 623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khattab, T.A.; Dacrory, S.; Abou-Yousef, H.; Kamel, S. Development of microporous cellulose-based smart xerogel reversible sensor via freeze drying for naked-eye detection of ammonia gas. Carbohydr. Polym. 2019, 210, 196–203. [Google Scholar] [CrossRef]
- Elanchezhian, C.; Ramnath, B.; Ramakrishnan, G.; Rajendrakumar, M.; Naveenkumar, V.; Saravanakumar, M. Review on mechanical properties of natural fiber composites. Mater. Today Proc. 2018, 5, 1785–1790. [Google Scholar] [CrossRef]
- Anuar, N.I.S.; Zakaria, S.; Gan, S.; Chia, C.H.; Wang, C.; Harun, J. Comparison of the morphological and mechanical properties of oil Palm EFB fibres and kenaf fibres in nonwoven reinforced composites. Ind. Crop. Prod. 2019, 127, 55–65. [Google Scholar] [CrossRef]
- Nguyen, H.; Zatar, W.; Mutsuyoshi, H. Mechanical Properties of Hybrid Polymer Composite; Elsevier Ltd.: Philadelphia, PA, USA, 2017; pp. 83–113. [Google Scholar]
- Ramamoorthy, S.K.; Skrifvars, M.; Persson, A. A Review of Natural Fibers Used in Biocomposites: Plant, Animal and Regenerated Cellulose Fibers. Polym. Rev. 2015, 55, 107–162. [Google Scholar] [CrossRef]
- Yu, M.-C.; Wan, J.-X. Environmental Friendly Development of Regenerated Cellulose Fiber Production. DEStech Trans. Eng. Technol. Res. 2017, 760–765. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Yan, Q.; Gao, X.; Wang, S.; He, Y.; Zhang, L. Infrared and fluorescence properties of reduced graphene oxide/regenerated cellulose composite fibers. BioResources 2020, 15, 4434–4448. [Google Scholar] [CrossRef]
- Nadhan, A.V.; Rajulu, A.V.; Li, R.; Jie, C.; Zhang, L. Properties of Regenerated Cellulose Short Fibers/Cellulose Green Composite Films. J. Polym. Environ. 2011, 20, 454–458. [Google Scholar] [CrossRef]
- Tian, M.; Qu, L.; Zhang, X.; Zhang, K.; Zhu, S.; Guo, X.; Han, G.; Tang, X.; Sun, Y. Enhanced mechanical and thermal properties of regenerated cellulose/graphene composite fibers. Carbohydr. Polym. 2014, 111, 456–462. [Google Scholar] [CrossRef]
- Ramamoorthy, S.K.; Kundu, C.K.; Adekunle, K.; Bashir, T.; Skrifvars, M.O.V. Properties of green composites with regenerated cellulose fiber and soybean-based thermoset for technical applications. J. Reinf. Plast. Compos. 2013, 33, 193–201. [Google Scholar] [CrossRef] [Green Version]
- Klar, V.; Orelma, H.; Rautkoski, H.; Kuosmanen, P.; Harlin, A. Spinning Approach for Cellulose Fiber Yarn Using a Deep Eutectic Solvent and an Inclined Channel. ACS Omega 2018, 3, 10918–10926. [Google Scholar] [CrossRef] [PubMed]
- De Silva, R.; Vongsanga, K.; Wang, X.; Byrne, N. Cellulose regeneration in ionic liquids: Factors controlling the degree of polymerisation. Cellulose 2015, 22, 2845–2849. [Google Scholar] [CrossRef]
- Orelma, H.; Hokkanen, A.; Leppänen, I.; Kammiovirta, K.; Kapulainen, M.; Harlin, A. Optical cellulose fiber made from regenerated cellulose and cellulose acetate for water sensor applications. Cellulose 2020, 27, 1543–1553. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Zhang, Y.; Dawelbeit, A.; Deng, Y.; Lang, Y.; Yu, M. Structure and properties of regenerated cellulose fibers from aqueous NaOH/thiourea/urea solution. Cellulose 2017, 24, 4123–4137. [Google Scholar] [CrossRef]
- Qiu, C.; Zhu, K.; Zhou, X.; Luo, L.; Zeng, J.; Huang, R.; Lu, A.; Liu, X.; Chen, F.; Zhang, L.; et al. Influences of Coagulation Conditions on the Structure and Properties of Regenerated Cellulose Filaments via Wet-Spinning in LiOH/Urea Solvent. ACS Sustain. Chem. Eng. 2018, 6, 4056–4067. [Google Scholar] [CrossRef]
- Jiang, G.; Yuan, Y.; Wang, B.; Yin, X.; Mukuze, K.S.; Huang, W.; Zhang, Y.; Wang, H. Analysis of regenerated cellulose fibers with ionic liquids as a solvent as spinning speed is increased. Cellulose 2012, 19, 1075–1083. [Google Scholar] [CrossRef]
- Lu, F.; Cheng, B.; Song, J.; Liang, Y. Rheological characterization of concentrated cellulose solutions in 1-allyl-3-methylimidazolium chloride. J. Appl. Polym. Sci. 2012, 124, 3419–3425. [Google Scholar] [CrossRef]
- Sun, H.; Miao, J.; Yu, Y.; Zhang, L. Dissolution of cellulose with a novel solvent and formation of regenerated cellulose fiber. Appl. Phys. A 2015, 119, 539–546. [Google Scholar] [CrossRef]
- Ma, Y.; Nasri-Nasrabadi, B.; You, X.; Wang, X.; Rainey, T.J.; Byrne, N. Regenerated Cellulose Fibers Wetspun from Different Waste Cellulose Types. J. Nat. Fibers 2020, 17, 1–13. [Google Scholar] [CrossRef]
- Uddin, A.J.; Yamamoto, A.; Gotoh, Y.; Nagura, M.; Iwata, M. Preparation and Physical Properties of Regenerated Cellulose Fibres from Sugarcane Bagasse. Text. Res. J. 2010, 80, 1846–1858. [Google Scholar] [CrossRef]
- Zhang, J.; Tominaga, K.; Yamagishi, N.; Gotoh, Y. Comparison of Regenerated Cellulose Fibers Spun from Ionic Liquid Solutions with Lyocell Fiber. J. Fiber Sci. Technol. 2020, 76, 257–266. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Salleh, W.W.; Jaafar, J.; Hir, Z.A.M.; Rosmi, M.S.; Mutalib, M.A.; Ismail, A.F.; Tanemura, M. Regenerated cellulose membrane as bio-template for in-situ growth of visible-light driven C-modified mesoporous titania. Carbohydr. Polym. 2016, 146, 166–173. [Google Scholar] [CrossRef]
- Xiong, X.; Duan, J.; Zou, W.; He, X.; Zheng, W. A pH-sensitive regenerated cellulose membrane. J. Membr. Sci. 2010, 363, 96–102. [Google Scholar] [CrossRef]
- Wang, S.; Lu, A.; Zhang, L. Recent advances in regenerated cellulose materials. Prog. Polym. Sci. 2016, 53, 169–206. [Google Scholar] [CrossRef]
- Czesław, Ś.; Beata, F. Structure—Property Relationships of Pure Cellulose and GO/CEL Membranes Regenerated from Ionic. Polymers 2019, 11, 1178. [Google Scholar]
- Mohamed, M.A.; Salleh, W.; Jaafar, J.; Ismail, A.F.; Mutalib, M.A.; Jamil, S.M. Incorporation of N-doped TiO2 nanorods in regenerated cellulose thin films fabricated from recycled newspaper as a green portable photocatalyst. Carbohydr. Polym. 2015, 133, 429–437. [Google Scholar] [CrossRef]
- Zheng, X.; Huang, F.; Chen, L.; Huang, L.; Cao, S.; Ma, X. Preparation of transparent film via cellulose regeneration: Correlations between ionic liquid and film properties. Carbohydr. Polym. 2019, 203, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, H.-C.; You, T.-T.; Wu, Y.-Y.; Ramaswamy, S.; Xu, F. Fabrication of regenerated cellulose membranes with high tensile strength and antibacterial property via surface amination. Ind. Crop. Prod. 2019, 140, 111603. [Google Scholar] [CrossRef]
- Le, N.L.; Nunes, S.P. Materials and membrane technologies for water and energy sustainability. Sustain. Mater. Technol. 2016, 7, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Boretti, A.; Rosa, L. Reassessing the projections of the World Water Development Report. NPJ Clean Water 2019, 2, 1–6. [Google Scholar] [CrossRef]
- Houngbo, G. Nature-Based Solutions for Water; UNESCO Publishing: Paris, France, 2018. [Google Scholar]
- Hu, M.-X.; Niu, H.-M.; Chen, X.-L.; Zhan, H.-B. Natural cellulose microfiltration membranes for oil/water nanoemulsions separation. Colloids Surf. A Physicochem. Eng. Asp. 2019, 564, 142–151. [Google Scholar] [CrossRef]
- Ao, C.; Hu, R.; Zhao, J.; Zhang, X.; Li, Q.; Xia, T.; Zhang, W.; Lu, C. Reusable, salt-tolerant and superhydrophilic cellulose hydrogel-coated mesh for efficient gravity-driven oil/water separation. Chem. Eng. J. 2018, 338, 271–277. [Google Scholar] [CrossRef]
- Halim, A.; Xu, Y.; Lin, K.-H.; Kobayashi, M.; Kajiyama, M.; Enomae, T. Fabrication of cellulose nanofiber-deposited cellulose sponge as an oil-water separation membrane. Sep. Purif. Technol. 2019, 224, 322–331. [Google Scholar] [CrossRef]
- Fan, T.; Qian, Q.; Hou, Z.; Liu, Y.; Lu, M. Preparation of smart and reversible wettability cellulose fabrics for oil/water separation using a facile and economical method. Carbohydr. Polym. 2018, 200, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Yagoub, H.; Zhu, L.; Shibraen, M.H.; Altam, A.A.; Babiker, D.M.; Rehan, K.; Mukwaya, V.; Xu, J.; Yang, S. Manipulating the surface wettability of polysaccharide based complex membrane for oil/water separation. Carbohydr. Polym. 2019, 225, 115231. [Google Scholar] [CrossRef] [PubMed]
- Ei-Kazzaz, A. Soilless Agriculture a New and Advanced Method for Agriculture Development: An Introduction. Agric. Res. Technol. Open Access J. 2017, 3, 555610. [Google Scholar] [CrossRef]
- Majeed, Z.; Ramli, N.K.; Mansor, N.; Man, Z. A comprehensive review on biodegradable polymers and their blends used in controlled-release fertilizer processes. Rev. Chem. Eng. 2015, 31, 69–95. [Google Scholar] [CrossRef]
- Sambo, P.; Nicoletto, C.; Giro, A.; Pii, Y.; Valentinuzzi, F.; Mimmo, T.; Lugli, P.; Orzes, G.; Mazzetto, F.; Astolfi, S.; et al. Hydroponic Solutions for Soilless Production Systems: Issues and Opportunities in a Smart Agriculture Perspective. Front. Plant Sci. 2019, 10, 923. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, B.; Deo, B. Soilless Farming–The Next Generation Green Revolution. Curr. Sci. 2019, 116, 728–732. [Google Scholar] [CrossRef]
- Muller, A.; Ferré, M.; Engel, S.; Gattinger, A.; Holzkämper, A.; Huber, R.; Müller, M.; Six, J. Can soil-less crop production be a sustainable option for soil conservation and future agriculture? Land Use Policy 2017, 69, 102–105. [Google Scholar] [CrossRef]
- Ranganathan, N.; Bensingh, R.J.; Kader, M.A.; Nayak, S.K. Cellulose-Based Hydrogels for Agricultures; Springer: Cham, Switzerland, 2019; pp. 1039–1059. [Google Scholar] [CrossRef]
- Kassem, I.; Kassab, Z.; Khouloud, M.; Sehaqui, H.; Bouhfid, R.; Jacquemin, J.; Qaiss, A.E.K.; El Achaby, M. Phosphoric acid-mediated green preparation of regenerated cellulose spheres and their use for all-cellulose cross-linked superabsorbent hydrogels. Int. J. Biol. Macromol. 2020, 162, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Luan, Q.; Huang, Q.; Tang, H.; Huang, F.; Li, W.; Wan, C.; Liu, C.; Xu, J.; Guo, P.; et al. A facile and efficient strategy for the fabrication of porous linseed gum/cellulose superabsorbent hydrogels for water conservation. Carbohydr. Polym. 2017, 157, 1830–1836. [Google Scholar] [CrossRef]
- Zainal, S.H.; Nofrianti, R.; Lazim, A.M.; Othaman, R. Cellulose-based hydrogel as Halal agricultural medium. Malays. Appl. Biol. 2019, 48, 109–113. [Google Scholar]
- Ibrahim, M.M.; Abd-Eladl, M.; Abou-Baker, N. Lignocellulosic biomass for the preparation of cellulose-based hydrogel and its use for optimizing water resources in agriculture. J. Appl. Polym. Sci. 2015, 132, 1–12. [Google Scholar] [CrossRef]
- Santoso, S.P.; Kurniawan, A.; Soetaredjo, F.E.; Cheng, K.-C.; Putro, J.; Ismadji, S.; Ju, Y.-H. Eco-friendly cellulose–bentonite porous composite hydrogels for adsorptive removal of azo dye and soilless culture. Cellulose 2019, 26, 3339–3358. [Google Scholar] [CrossRef]
- Rosa, L.; Chiarelli, D.D.; Rulli, M.C.; Dell’Angelo, J.; D’Odorico, P. Global agricultural economic water scarcity. Sci. Adv. 2020, 6, eaaz6031. [Google Scholar] [CrossRef]
- Abou-Baker, N.; Ouis, M.; Abd-Eladl, M.; Ibrahim, M.M. Transformation of Lignocellulosic Biomass to Cellulose-Based Hydrogel and Agriglass to Improve Beans Yield. Waste Biomass Valorization 2019, 11, 3537–3551. [Google Scholar] [CrossRef]
- Ciolacu, D.; Ciolacu, F.; Popa, V.I. Amorphous Cellulose—Structure and Characterization. Cellulose Chem. Technol. 2011, 45, 13–21. [Google Scholar]
- Ling, Z.; Chen, S.; Zhang, X.; Takabe, K.; Xu, F. Unraveling variations of crystalline cellulose induced by ionic liquid and their effects on enzymatic hydrolysis. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Halonen, H.; Larsson, P.T.; Iversen, T. Mercerized cellulose biocomposites: A study of influence of mercerization on cellulose supramolecular structure, water retention value and tensile properties. Cellulose 2013, 20, 57–65. [Google Scholar] [CrossRef]
- Khazraji, A.C.; Robert, S. Interaction Effects between Cellulose and Water in Nanocrystalline and Amorphous Regions: A Novel Approach Using Molecular Modeling. J. Nanomater. 2013, 2013, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.; Duan, B.; Cai, J.; Zhang, L. Superabsorbent hydrogels based on cellulose for smart swelling and controllable delivery. Eur. Polym. J. 2010, 46, 92–100. [Google Scholar] [CrossRef]
- Demitri, C.; Scalera, F.; Madaghiele, M.; Sannino, A.; Maffezzoli, A. Potential of Cellulose-Based Superabsorbent Hydrogels as Water Reservoir in Agriculture. Int. J. Polym. Sci. 2013, 2013, 1–6. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, M.; Luan, Q.; Tang, H.; Huang, F.; Xiang, X.; Yang, C.; Bao, Y. Cellulose Anionic Hydrogels Based on Cellulose Nanofibers as Natural Stimulants for Seed Germination and Seedling Growth. J. Agric. Food Chem. 2017, 65, 3785–3791. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, D.; Bai, X.; Zhang, W.; Lu, X. Identification and Characterization of a Large Protein Essential for Degradation of the Crystalline Region of Cellulose by Cytophaga hutchinsonii. Appl. Environ. Microbiol. 2017, 83, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Mondéjar, R.; Zühlke, D.; Becher, D.; Riedel, K.; Baldrian, P. Cellulose and hemicellulose decomposition by forest soil bacteria proceeds by the action of structurally variable enzymatic systems. Sci. Rep. 2016, 6, 25279. [Google Scholar] [CrossRef]
- Leppänen, I.; Vikman, M.; Harlin, A.; Orelma, H. Enzymatic Degradation and Pilot-Scale Composting of Cellulose-Based Films with Different Chemical Structures. J. Polym. Environ. 2019, 28, 458–470. [Google Scholar] [CrossRef] [Green Version]
- Pan, Y.; Zhao, X.; Li, X.; Cai, P. Green-Based Antimicrobial Hydrogels Prepared from Bagasse Cellulose as 3D-Scaffolds for Wound Dressing. Polymers 2019, 11, 1846. [Google Scholar] [CrossRef] [Green Version]
- Edgar, K.J.; Zhang, H. Antibacterial modification of Lyocell fiber: A review. Carbohydr. Polym. 2020, 250, 116932. [Google Scholar] [CrossRef]
- Wu, H.; Hu, S.; Nie, C.; Zhang, J.; Tian, H.; Hu, W.; Shen, T.; Wang, J. Fabrication and characterization of antibacterial epsilon-poly-L-lysine anchored dicarboxyl cellulose beads. Carbohydr. Polym. 2021, 255, 117337. [Google Scholar] [CrossRef]
- Nie, C.; Shen, T.; Hu, W.; Ma, Q.; Zhang, J.; Hu, S.; Tian, H.; Wu, H.; Luo, X.; Wang, J. Characterization and antibacterial properties of epsilon-poly- l-lysine grafted multi-functional cellulose beads. Carbohydr. Polym. 2021, 262, 117902. [Google Scholar] [CrossRef]
- Rkhaila, A.; Chtouki, T.; Erguig, H.; El Haloui, N.; Ounine, K. Chemical Proprieties of Biopolymers (Chitin/Chitosan) and Their Synergic Effects with Endophytic Bacillus Species: Unlimited Applications in Agriculture. Molecules 2021, 26, 1117. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Duan, J.; Xu, Q.; Wei, X.; Lu, A.; Zhang, L. Ampholytic microspheres constructed from chitosan and carrageenan in alkali/urea aqueous solution for purification of various wastewater. Chem. Eng. J. 2017, 317, 766–776. [Google Scholar] [CrossRef]
- Xu, Q.; Ji, Y.; Sun, Q.; Fu, Y.; Xu, Y.; Jin, L. Fabrication of Cellulose Nanocrystal/Chitosan Hydrogel for Controlled Drug Release. Nanomaterial 2019, 9, 253. [Google Scholar] [CrossRef] [PubMed]
- Rop, K.; Karuku, G.N.; Mbui, D.; Michira, I.; Njomo, N. Formulation of slow release NPK fertilizer (cellulose-graft-poly(acrylamide)/nano-hydroxyapatite/soluble fertilizer) composite and evaluating its N mineralization potential. Ann. Agric. Sci. 2018, 63, 163–172. [Google Scholar] [CrossRef]
- Ramli, R.A. Slow release fertilizer hydrogels: A review. Polym. Chem. 2019, 10, 6073–6090. [Google Scholar] [CrossRef]
- Naz, M.Y.; Sulaiman, S.A. Slow release coating remedy for nitrogen loss from conventional urea: A review. J. Control. Release 2016, 225, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Sempeho, S.I.; Kim, H.T.; Mubofu, E.; Hilonga, A. Meticulous Overview on the Controlled Release Fertilizers. Adv. Chem. 2014, 2014, 1–16. [Google Scholar] [CrossRef]
- Davidson, D.W.; Verma, M.S.; Gu, F.X. Controlled root targeted delivery of fertilizer using an ionically crosslinked carboxymethyl cellulose hydrogel matrix. SpringerPlus 2013, 2, 318. [Google Scholar] [CrossRef] [Green Version]
- Septevani, A.A.; Rifathin, A.; Sari, A.A.; Sampora, Y.; Ariani, G.N.; Sudiyarmanto, A.; Sondari, D. Oil palm empty fruit bunch-based nanocellulose as a super-adsorbent for water remediation. Carbohydr. Polym. 2020, 229, 115433. [Google Scholar] [CrossRef]
- Guilherme, M.R.; Aouada, F.; Fajardo, A.; Martins, A.F.; Paulino, A.; Davi, M.F.; Rubira, A.; Muniz, E. Superabsorbent hydrogels based on polysaccharides for application in agriculture as soil conditioner and nutrient carrier: A review. Eur. Polym. J. 2015, 72, 365–385. [Google Scholar] [CrossRef] [Green Version]
- Ramli, R.A.; Lian, Y.M.; Nor, N.M.; Azman, N.I.Z. Synthesis, characterization, and morphology study of coco peat-grafted-poly(acrylic acid)/NPK slow release fertilizer hydrogel. J. Polym. Res. 2019, 26, 266. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Lu, P.; Zhang, M. Preparation and properties of hydrogel based on sawdust cellulose for environmentally friendly slow release fertilizers. Green Process. Synth. 2020, 9, 139–152. [Google Scholar] [CrossRef]
- Olad, A.; Zebhi, H.; Salari, D.; Mirmohseni, A.; Tabar, A.R. Water retention and slow release studies of a salep-based hydrogel nanocomposite reinforced with montmorillonite clay. N. J. Chem. 2018, 42, 2758–2766. [Google Scholar] [CrossRef]
- Li, X.; Li, Q.; Xu, X.; Su, Y.; Yue, Q.; Gao, B. Characterization, swelling and slow-release properties of a new controlled release fertilizer based on wheat straw cellulose hydrogel. J. Taiwan Inst. Chem. Eng. 2016, 60, 564–572. [Google Scholar] [CrossRef]
- Aini, A.K.; Hamzah, R.; Noriman, N.Z.; AlRashdi, A.; Johari, I.; Razlan, Z.M.; Shahriman, A.B.; Zunaidi, I.; Khairunizam, W. Slow Release Fertilizer from Treated Rice Straw/Urea Beads Coated With Natural Rubber: FTIR and UV-Vis Analysis. IOP Conf. Ser. Mater. Sci. Eng. 2019, 557, 012068. [Google Scholar] [CrossRef]
- Rashidzadeh, A.; Olad, A. Slow-released NPK fertilizer encapsulated by NaAlg-g-poly(AA-co-AAm)/MMT superabsorbent nanocomposite. Carbohydr. Polym. 2014, 114, 269–278. [Google Scholar] [CrossRef]
- He, Y.; Wu, Z.; Tu, L.; Han, Y.; Zhang, G.; Li, C. Encapsulation and characterization of slow-release microbial fertilizer from the composites of bentonite and alginate. Appl. Clay Sci. 2015, 109, 68–75. [Google Scholar] [CrossRef]
- Wu, Z.; He, Y.; Chen, L.; Han, Y.; Li, C. Characterization of Raoultella planticola Rs-2 microcapsule prepared with a blend of alginate and starch and its release behavior. Carbohydr. Polym. 2014, 110, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Abbah, S.A.; Saran, K.; Zhang, Y.; Li, J.; Wong, H.-K.; Goh, J.C.H. Silk Fibroin-Based Complex Particles with Bioactive Encrustation for Bone Morphogenetic Protein 2 Delivery. Biomacromolecules 2013, 14, 4465–4474. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi-Khoo, S.; Moghadam, P.N.; Fareghi, A.R.; Movagharnezhad, N. Synthesis of a cellulose-based hydrogel network: Characterization and study of urea fertilizer slow release. J. Appl. Polym. Sci. 2016, 133, 1–9. [Google Scholar] [CrossRef]
- Olad, A.; Zebhi, H.; Salari, D.; Mirmohseni, A.; Tabar, A.R. Slow-release NPK fertilizer encapsulated by carboxymethyl cellulose-based nanocomposite with the function of water retention in soil. Mater. Sci. Eng. C 2018, 90, 333–340. [Google Scholar] [CrossRef] [PubMed]
- El Sayed, A.M.; El-Gamal, S.; Morsi, W.M.; Mohammed, G. Effect of PVA and copper oxide nanoparticles on the structural, optical, and electrical properties of carboxymethyl cellulose films. J. Mater. Sci. 2015, 50, 4717–4728. [Google Scholar] [CrossRef]
- Nayan, N.H.M.; Hamzah, M.S.A.; Tahir, A.A.-H.M.; Rajali, A.A.A.; Muslih, E.F.; Mazlan, R. Development of Polyvinyl Alcohol/Chitosan Hydrogel Loaded with Fertilizer Compound: Preparation, Properties and Effect on Seed Germination. J. Sci. Technol. 2018, 10, 21–27. [Google Scholar] [CrossRef] [Green Version]
- Kasirajan, S.; Ngouajio, M. Polyethylene and biodegradable mulches for agricultural applications: A review. Agron. Sustain. Dev. 2012, 32, 501–529. [Google Scholar] [CrossRef]
- Zribi, W.; Aragüés, R.; Medina, E.; Faci, J. Efficiency of inorganic and organic mulching materials for soil evaporation control. Soil Tillage Res. 2015, 148, 40–45. [Google Scholar] [CrossRef] [Green Version]
- Kader, A.; Senge, M.; Mojid, M.; Ito, K. Recent advances in mulching materials and methods for modifying soil environment. Soil Tillage Res. 2017, 168, 155–166. [Google Scholar] [CrossRef]
- Bandopadhyay, S.; Martin-Closas, L.; Pelacho, A.; Debruyn, J.M. Biodegradable Plastic Mulch Films: Impacts on Soil Microbial Communities and Ecosystem Functions. Front. Microbiol. 2018, 9, 819. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Dai, R.; Shan, Z.; Chen, H. Fabrication and characterization of one high-hygroscopicity liquid starch-based mulching materials for facilitating the growth of plant. Carbohydr. Polym. 2020, 230, 115582. [Google Scholar] [CrossRef]
- Zhang, M.; Dong, B.; Qiao, Y.; Yang, H.; Wang, Y.; Liu, M. Effects of sub-soil plastic film mulch on soil water and salt content and water utilization by winter wheat under different soil salinities. Field Crop. Res. 2018, 225, 130–140. [Google Scholar] [CrossRef]
- Sun, T.; Li, G.; Ning, T.-Y.; Zhang, Z.-M.; Mi, Q.-H.; Lal, R. Suitability of mulching with biodegradable film to moderate soil temperature and moisture and to increase photosynthesis and yield in peanut. Agric. Water Manag. 2018, 208, 214–223. [Google Scholar] [CrossRef]
- Gang, X.; Huabing, L.; Yufu, P.; Tiezhao, Y.; Xi, Y.; Shixiao, X. Plastic film mulching combined with nutrient management to improve water use efficiency, production of rain-fed maize and economic returns in semi-arid regions. Field Crop. Res. 2019, 231, 30–39. [Google Scholar] [CrossRef]
- Kader, M.A.; Singha, A.; Begum, M.A.; Jewel, A.; Khan, F.H.; Khan, N.I. Mulching as water-saving technique in dryland agriculture: Review article. Bull. Natl. Res. Cent. 2019, 43, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Chopra, M.; Koul, B. Comparative assessment of different types of mulching in various crops: A review. Plant Arch. 2020, 20, 1620–1626. [Google Scholar]
- Sivakumar, D.; Jifon, J. Influence of Photoselective Shade Nettings on Postharvest Quality of Vegetables; Elsevier Inc.: Philadelphia, PA, USA, 2018; pp. 121–138. [Google Scholar]
- Briassoulis, D.; Mistriotis, A.; Eleftherakis, D. Mechanical behaviour and properties of agricultural nets—Part I: Testing methods for agricultural nets. Polym. Test 2007, 26, 822–832. [Google Scholar] [CrossRef]
- Scarascia-Mugnozza, G.; Sica, C.; Russo, G. Plastic materials in European agriculture: Actual use and perspectives. J. Agric. Eng. 2012, 42, 15–28. [Google Scholar] [CrossRef]
- Castellano, S.; Mugnozza, G.S.; Russo, G.; Briassoulis, D.; Mistriotis, A.; Hemming, S.; Waaijenberg, D. Plastic nets in agriculture: A general review of types and applications. Appl. Eng. Agric. 2008, 24, 799–808. [Google Scholar] [CrossRef]
- Divya, V.; Sarkar, N. Plastic Mulch Pollution and Introduction of Biodegradable Plastic Mulches—A Review. Agric. Rev. 2019, 40, 314–318. [Google Scholar] [CrossRef]
- Ghimire, S.; Scheenstra, E.; Miles, C.A. Soil-biodegradable Mulches for Growth, Yield, and Quality of Sweet Corn in a Mediterranean-type Climate. HortScience 2020, 55, 317–325. [Google Scholar] [CrossRef] [Green Version]
- Cirujeda, A.; Aibar, J.; Anzalone, Á.; Martin-Closas, L.; Meco, R.; Moreno, M.M.; Pardo, A.; Pelacho, A.; Rojo, F.; Royo-Esnal, A.; et al. Biodegradable mulch instead of polyethylene for weed control of processing tomato production. Agron. Sustain. Dev. 2012, 32, 889–897. [Google Scholar] [CrossRef] [Green Version]
- Cao, L.; Liu, C.; Zou, D.; Zhang, S.; Chen, Y. Using cellulose nanocrystals as sustainable additive to enhance mechanical and shape memory properties of PLA/ENR thermoplastic vulcanizates. Carbohydr. Polym. 2020, 230, 115618. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, L.; Tian, H.; Lu, A. Universal preparation of cellulose-based colorimetric sensor for heavy metal ion detection. Carbohydr. Polym. 2020, 236, 116037. [Google Scholar] [CrossRef]
- Soni, B.; Hassan, E.B.; Schilling, M.W.; Mahmoud, B. Transparent bionanocomposite films based on chitosan and TEMPO-oxidized cellulose nanofibers with enhanced mechanical and barrier properties. Carbohydr. Polym. 2016, 151, 779–789. [Google Scholar] [CrossRef] [Green Version]
- Ning, R.; Liang, J.; Sun, Z.; Liu, X.; Sun, W. Preparation and characterization of black biodegradable mulch films from multiple biomass materials. Polym. Degrad. Stab. 2021, 183, 109411. [Google Scholar] [CrossRef]
- Mendonça, S.R.; Ávila, M.C.R.; Vital, R.G.; Evangelista, Z.R.; Pontes, N.D.C.; Nascimento, A.D.R. The effect of different mulching on tomato development and yield. Sci. Hortic. 2021, 275, 109657. [Google Scholar] [CrossRef]
- Sarkar, D.; Solaiman, A.H.M.; Jahan, M.S.; Rojoni, R.N.; Kabir, K.; Hasanuzzaman, M. Soil parameters, onion growth, physiology, biochemical and mineral nutrient composition in response to colored polythene film mulches. Ann. Agric. Sci. 2019, 64, 63–70. [Google Scholar] [CrossRef]
- Franquera, E.N.; Mabesa, R.C. Colored Plastic Mulch Effects on the Yield of Lettuce (Lactuca sativa L.) and Soil Temperature. J. Adv. Agric. Technol. 2016, 3, 155–159. [Google Scholar] [CrossRef]
- Sintim, H.Y.; Bary, A.I.; Hayes, D.G.; Wadsworth, L.C.; Anunciado, M.B.; English, M.E.; Bandopadhyay, S.; Schaeffer, S.M.; DeBruyn, J.; Miles, C.A.; et al. In situ degradation of biodegradable plastic mulch films in compost and agricultural soils. Sci. Total. Environ. 2020, 727, 138668. [Google Scholar] [CrossRef]
- Yang, Y.; Li, P.; Jiao, J.; Yang, Z.; Lv, M.; Li, Y.; Zhou, C.; Wang, C.; He, Z.; Liu, Y.; et al. Renewable sourced biodegradable mulches and their environment impact. Sci. Hortic. 2020, 268, 109375. [Google Scholar] [CrossRef]
- Gupta, G.S. Land Degradation and Challenges of Food Security. Rev. Eur. Stud. 2019, 11, p63. [Google Scholar] [CrossRef]
- Chae, Y.; An, Y.-J. Current research trends on plastic pollution and ecological impacts on the soil ecosystem: A review. Environ. Pollut. 2018, 240, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Abhilash, M.; Thomas, D. Biopolymers for biocomposites and chemical sensor applications. In Biopolymer Composites in Electronics; Elsevier Inc.: Philadelphia, PA, USA, 2017; pp. 405–435. [Google Scholar] [CrossRef]
- Schaude, C.; Meindl, C.; Fröhlich, E.; Attard, J.; Mohr, G.J. Developing a sensor layer for the optical detection of amines during food spoilage. Talanta 2017, 170, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Shahi, N.; Joshi, G.; Min, B. Effect of Regenerated Cellulose Fibers Derived from Black Oat on Functional Properties of PVA-Based Biocomposite Film. Processes 2020, 8, 1149. [Google Scholar] [CrossRef]
- Brinkhoff, J.; Hornbuckle, J.; Dowling, T. Multisensor Capacitance Probes for Simultaneously Monitoring Rice Field Soil-Water-Crop-Ambient Conditions. Sensors 2017, 18, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Zhang, L.; Zhou, J.; Lu, A. Flexible and Transparent Cellulose-Based Ionic Film as a Humidity Sensor. ACS Appl. Mater. Interfaces 2020, 12, 7631–7638. [Google Scholar] [CrossRef] [PubMed]







| Type of Solvent | Name of Solvent | Formation of Intermediates | Reaction Mechanism |
|---|---|---|---|
| Derivatizing | N,N-dimethylformamide (DMF)/dinitrogen tetraoxide (N2O4) | Etherification reaction occurs at O6 and O2 that forms cellulose I into cellulose nitrite arose from N2O4 as the intermediate preceding the dissolution as DMF acts as the organic solvent. Common catalysts such as sulphuric acid or phosphoric acid are involved in this reaction. |  |
| Derivatizing | Trifluoroacetic acid | Cellulose triacetate is formed through esterification reaction at O2 and O6 as derivatives before dissolution takes place. |  |
| Non-derivatizing | N-methylmorpholine-N-oxide (NMMO)/water (aqueous system) | Widely used as the industrial cellulose solvent, the highly polar N-O bond is able to form one or two hydrogen bond(s) with two hydroxyl groups in cellulose. The molecule of H2O that is smaller than NMMO also aids in breaking the inter- and intramolecular hydrogen bonding [25]. |  |
| Non-derivatizing | Sodium hydroxide (NaOH)/urea/water (aqueous system) | Cellulose chain degradation is caused by alkaline hydrolysis. NaOH is interfering with inter- and intramolecular hydrogen bonding of O3H--O5′, O2H---O6′, and O3′---O6H during the process of mercerization of cellulose [37]. |  |
| Non-derivatizing | 1-butyl-3-methylimizadolium chloride [BMIM]Cl (non-aqueous system) | IL that is derived from cationic BMIM+ and anionic Cl- caused the charges are distant due to bulky ‘shell’ around the BMIM+. Cl- forms bonds with H+ in the hydroxyl group of cellulose to interfere with the supramolecular structure. |  |
| Characterization | Method | Cellulose I | Cellulose II | Explanation |
|---|---|---|---|---|
| Crystalline structure | XRD | Crystalline interplanar (1–10), (110), (012), (200), (004) Miller indices and amorphous are represented by 12.3°, 16.5°, 20.4°, 22.6°, 34.4° and near 20.5° [42,43]. | Crystalline interplanar of (1–10), (110), (020) and amorphous shown in XRD profile of cellulose II that are represented by ~12.3°, ~20.1°, ~22.0° and ~20.5°. Overlap (110) and (020) peaks might be attributable for more amorphous RC subjected to type and parameter of cellulose solvent [42,44]. | The loss of peak and generation of the crystalline peaks are due to the rebuilding of hydrogen bonding [42]. |
| FTIR | 1428 cm−1 peak is considered to be the crystalline region [45,46] | The 1428 cm−1 peak is lost [42]. | ||
| C13 CP-MAS NMR | Singlet signal 100–110 ppm, 96 ppm, and 60–70 ppm correspond to C1, C1 (reducing end), and C6 [47] | Extra small shoulder peak at 107 ppm, peak of C1 reducing end at 96 ppm is not detected, and C6 peak is shifted [47] | C6 peak confirms the crystalline structure of cellulose, where, when it is not detected in cellulose II, it is shown that cellulose has a lower degree of polymerization compared to cellulose I. | |
| Chemical structure | FTIR | O-H stretching, C-H2 stretching, and C-O-C pyranose ring vibrations at 3700–3300 cm−1, 2900 cm−1, and 1163 cm−1 [42,48] | O-H stretching, C-H2 stretching, and C-O-C pyranose ring vibrations at 3345–3393 cm−1 (shift to a higher number), 2921 cm−1 (shift to a higher number and 1157 cm−1 (shift to a lower number) [42]. | Transformation of cellulose I to cellulose II has occurred. O-H peak is shifted to a lower number is due to more –OH are created and more water adsorption [48]. |
| Decomposition | Thermogravimetric analysis (TGA) | Tonset that refers to the removal of water is higher while the decomposition of cellulose reflected by Tmax is lower [42,49,50]. | In contrast, Tonset that refers to the removal of water is lower while the decomposition of cellulose reflected by Tmax is higher [42,49,50]. | The lower Tonset is due to the lower crystallinity recorded in cellulose II. Cellulose II is more thermally stable than cellulose I is due to its more ordered antiparallel chain that is regenerated after dissolution. |
| Differential scanning calorimetry (DSC) | Has higher Tg = 64 ℃ [51] | Lower Tg = 62 ℃ [51] | More –OH surface groups on cellulose I |
| Source of Cellulose | Type of Solvent | Coagulation Bath | Properties of RC Fibers | References |
|---|---|---|---|---|
| Sugarcane Bagasse | N-methylmorpholine-N-oxide (NMMO) hydrate | Ethanol | Morphology: Higher fibrillation Elongation at break: 2% Tensile strength: 530 MPa | [89] |
| Dissolving pulp | 1-butyl-3-methylimidazolium chloride (BMIMCl) | Water | Morphology: No obvious voids and intact surface with dense inner structure Elongation at break: 6.6% Tensile strength: 808 MPa | [90] |
| Wood pulp | 1-butyl-3-methylimidazolium acetate ((Bmim)OAc)/dimethyl sulfoxide (DMSO) | Water | Morphology: Irregular, serrated cross-section and severe grooves of the surface Elongation at break: 5.4% Tensile strength: 22.5 cN/tex | [88] |
| Soft wood pulp | Tetrabutylammonium acetate (TBAA)/dimethyl sulfoxide (DMSO) | Acid | Morphology: Smooth surface as round and compact structure Elongation at break: 9.7% Tensile strength: 2.15 cN/dtex | [87] |
| Cotton linters | NaOH/thiourea/urea | Acid | Morphology: Smooth surface and circular cross-section with homogeneous and denser structure Elongation at break: 9.47% Tensile strenghth: 2.22 cN/dtex | [83] |
| Type | Material | Classification | Method | Crosslinker | Swelling Capacity | Porosity | Application | Reference |
|---|---|---|---|---|---|---|---|---|
| Hydrogel film composite | Cellulose/gelatin | Natural | Dissolution-regeneration process with IL and distilled water | Photocrosslinked by high UV at different time length | Higher with longer time length | - | High water retention ability | [59] |
| Superabsorbent composite hydrogel film | Microcrystalline cellulose/carboxymethyl cellulose/hydroxyethyl cellulose | Natural | Dissolution-regeneration process using cold phosphoric acid and regenerate by using water | Citric acid at 80 ℃ | Approximately 4000% | - | Coating material for control release fertilizer | [112] |
| Hydrophilic Composite hydrogel | Cellulose/linseed gum | Natural | Dissolution-regeneration process with NaOH/urea and regenerated by distilled water | Epichlorohydrin at 60 ℃ 30 min | more than 330 g/g | 28 µm | Water conserver to be mixed with soil | [113] |
| Hydrogel | Cellulose | Natural | Dissolution-regeneration process with NaOH/urea system | Citric acid at varied temperature | Increase with increasing cross-linking temperature, more than 20% | - | Cultivation culture media for plantation | [114] |
| Grafted SAH | Cellulose/polyacrylic acid | Natural-synthetic biodegradable | Dissolution-regeneration process by using activated cellulose which are then dissolved and washed in distilled water. | N,N-methyle-nebisacrylamide | More than 3000% | More open and loose structure | Soil optimization to increase efficiency in seed cultivation | [115] |
| Composite hydrogel | Cellulose/bentonite | Natural | Dissolution-regeneration process using NaOH/urea as a solvent and regenerate using distilled water | Epichlorohydrin | 5.74 g/g | - | Promoting seed growth and adsorption in soil | [116] |
| Hydrogel | Wheat straw cellulose | Natural | Dissolution-regeneration process by dissolving in NaOH and regenerated using ethanol. | N,N-methyle-nebisacrylamide | 3095.74% swelling capacity with intermediate cellulose concentration | - | Soil has high water retention and improving seed growth and germination | [54] |
| RC Hydrogels | Source of Cellulose | Diffusional Exponent, n | Release Rate Constant, k | Correlation Coefficient, R2 | Model | Nutrient Release (%) Per Time | References |
|---|---|---|---|---|---|---|---|
| Sawdust cellulose-grafting-poly(acrylic acid)-poly(acrylamide)-Urea | Sawdust | 1.3214 | 0.0012 | 0.9781 | Ritger-Peppas | 2.4% per 10 min 8.6% per 30 min 82.4% per 480 min | [144] |
| Cellulose hydrogel-Urea | Cellulose microcrystal | 1.4476 | 0.2296 | 0.9899 | Ritger-Peppas | 22.9% per 1 day 49.5% per 3 days 75.6% per 6 days 91.3% per 10 days 95.71% per 21 days | [152] |
| Salep-g-poly(acrylic acid)/montmorillonite clay and NPK | Salep | 0.3371 | 0.3004 | 0.9887 | Korsmeyer-Peppas | 14.12% per a day 30.32% per a week 55.36% per a month | [145] |
| Sulfonated-carboxymethyl cellulose with acrylic acid in polyvinylpyrrolidone, silica nanoparticles and NPK | Carboxymethyl cellulose | 0.3570 | 0.3423 | 0.9862 | Korsmeyer-Peppas | 14.6% per a day 27.6% per a week 54.6% per a month | [153] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zainul Armir, N.A.; Zulkifli, A.; Gunaseelan, S.; Palanivelu, S.D.; Salleh, K.M.; Che Othman, M.H.; Zakaria, S. Regenerated Cellulose Products for Agricultural and Their Potential: A Review. Polymers 2021, 13, 3586. https://doi.org/10.3390/polym13203586
Zainul Armir NA, Zulkifli A, Gunaseelan S, Palanivelu SD, Salleh KM, Che Othman MH, Zakaria S. Regenerated Cellulose Products for Agricultural and Their Potential: A Review. Polymers. 2021; 13(20):3586. https://doi.org/10.3390/polym13203586
Chicago/Turabian StyleZainul Armir, Nur Amira, Amalia Zulkifli, Shamini Gunaseelan, Swarna Devi Palanivelu, Kushairi Mohd Salleh, Muhamad Hafiz Che Othman, and Sarani Zakaria. 2021. "Regenerated Cellulose Products for Agricultural and Their Potential: A Review" Polymers 13, no. 20: 3586. https://doi.org/10.3390/polym13203586