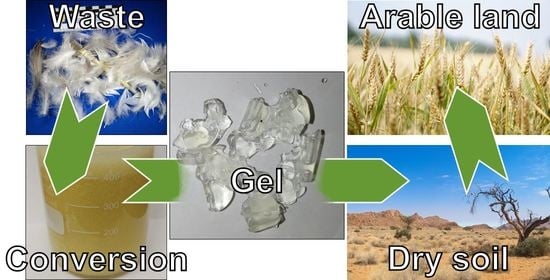
Autogenous Cross-Linking of Recycled Keratin from Poultry-Feather Waste to Hydrogels for Plant-Growth Media
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Keratin Hydrolysate from Feathers under Reducing Conditions
2.3. Preparations of Keratin Gels
2.4. Swelling Experiments
2.5. Elemental Analysis
2.6. Sulphur and Carbon Analysis
2.7. Amino Acid Analysis
2.8. SDS-PAGE Analysis of Extracted Keratin
2.9. Infrared Spectroscopy (FTIR-ATR)
3. Results and Discussion
3.1. Feather Keratin Hydrolysis with Sodium Sulphide
3.2. Keratin Hydrogel Formation by Drying
3.3. Influence of Drying Conditions
3.4. Prove-of-Principle Experiment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, W.-L.; Chen, M.-Y.; Tu, I.F.; Lin, Y.-C.; Eswar Kumar, N.; Chen, M.-Y.; Ho, M.-C.; Wu, S.-H. The discovery of novel heat-stable keratinases from Meiothermus taiwanensis WR-220 and other extremophiles. Sci. Rep. 2017, 7, 4658. [Google Scholar] [CrossRef] [Green Version]
- McKittrick, J.; Chen, P.Y.; Bodde, S.G.; Yang, W.; Novitskaya, E.E.; Meyers, M.A. The Structure, Functions, and Mechanical Properties of Keratin. JOM 2012, 64, 449–468. [Google Scholar] [CrossRef]
- Wang, B.; Yang, W.; McKittrick, J.; Meyers, M.A. Keratin: Structure, mechanical properties, occurrence in biological organisms, and efforts at bioinspiration. Prog. Mater. Sci. 2016, 76, 229–318. [Google Scholar] [CrossRef] [Green Version]
- Reddy, N. Keratin-based Biomaterials and Bioproducts, 1st ed.; Smithers Rapra Technology: Shawbury, UK, 2017. [Google Scholar]
- Bear, R.S.; Rugo, H.J. The Results of X-ray Diffraction Studies on Keratin Fibers. Ann. N. Y. Acad. Sci. 1951, 53, 627–648. [Google Scholar] [CrossRef] [PubMed]
- Parry, D.A.; North, A. Hard α-keratin intermediate filament chains: Substructure of the N-and C-terminal domains and the predicted structure and function of the C-terminal domains of type I and type II chains. J. Struct. Biol. 1998, 122, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Goddard, D.R.; Michaelis, L. A Study on Keratin. J. Biol. Chem. 1934, 106, 605–614. [Google Scholar] [CrossRef]
- Grazziotin, A.; Pimentel, F.; De Jong, E.; Brandelli, A. Nutritional improvement of feather protein by treatment with microbial keratinase. Anim. Feed Sci. Technol. 2006, 126, 135–144. [Google Scholar] [CrossRef]
- Onifade, A.; Al-Sane, N.; Al-Musallam, A.; Al-Zarban, S. A review: Potentials for biotechnological applications of keratin-degrading microorganisms and their enzymes for nutritional improvement of feathers and other keratins as livestock feed resources. Bioresour. Technol. 1998, 66, 1–11. [Google Scholar] [CrossRef]
- Barone, J.R.; Schmidt, W.F. Polyethylene reinforced with keratin fibers obtained from chicken feathers. Compos. Sci. Technol. 2005, 65, 173–181. [Google Scholar] [CrossRef]
- Aranberri, I.; Montes, S.; Azcune, I.; Rekondo, A.; Grande, H.-J. Fully Biodegradable Biocomposites with High Chicken Feather Content. Polymers 2017, 9, 593. [Google Scholar] [CrossRef] [Green Version]
- Barone, J.R.; Schmidt, W.F.; Liebner, C.F.E. Thermally processed keratin films. J. Appl. Polym. Sci. 2005, 97, 1644–1651. [Google Scholar] [CrossRef]
- Barone, J.R.; Schmidt, W.F.; Gregoire, N. Extrusion of feather keratin. J. Appl. Polym. Sci. 2006, 100, 1432–1442. [Google Scholar] [CrossRef]
- Ullah, A.; Wu, J. Feather Fiber-Based Thermoplastics: Effects of Different Plasticizers on Material Properties. Macromol. Mater. Eng. 2012, 298, 153–162. [Google Scholar] [CrossRef]
- Brenner, M.; Popescu, C.; Weichold, O. Anti-Frothing Effect of Poultry Feathers in Bio-Based, Polycondensation-Type Thermoset Composites. Appl. Sci. 2020, 10, 2150. [Google Scholar] [CrossRef] [Green Version]
- Hadas, A.; Kautsky, L. Feather meal, a semi-slow-release nitrogen fertilizer for organic farming. Fertil. Res. 1994, 38, 165–170. [Google Scholar] [CrossRef]
- Joardar, J.C.; Rahman, M.M. Poultry feather waste management and effects on plant growth. Int. J. Recycl. Org. Waste Agric. 2018, 7, 183–188. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.-M.; Nelson, P.V. Developing a slow-release nitrogen fertilizer from organic sources: II. Using poultry feathers. J. Am. Soc. Hortic. Sci. 1996, 121, 634–638. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.-M.; Nelson, P.V. Developing a slow-release nitrogen fertilizer from organic sources: III. Isolation and action of a feather-degrading actinomycete. J. Am. Soc. Hortic. Sci. 1996, 121, 639–643. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Liebeck, B.M.; Yan, K.; Demco, D.E.; Körner, A.; Popescu, C. Alpha-Helix Self-Assembly of Oligopeptides Originated from Beta-Sheet Keratin. Macromol. Chem. Phys. 2012, 213, 2628–2638. [Google Scholar] [CrossRef]
- Nagai, Y.; Nishikawa, T. Alkali Solubilization of Chicken Feather Keratin. Agric. Biol. Chem. 1970, 34, 16–22. [Google Scholar] [CrossRef]
- Crewther, W.; Fraser, R.; Lennox, F.; Lindley, H. The chemistry of keratins. In Advances in Protein Chemistry; Elsevier: Amsterdam, The Netherlands, 1965; Volume 20, pp. 191–346. [Google Scholar]
- Asquith, R.; Leon, N. Chemical reactions of keratin fibers. In Chemistry of Natural Protein Fibers; Springer: Berlin, Germany, 1977; pp. 193–265. [Google Scholar]
- Shih, J.C. Recent development in poultry waste dizgestion and feather utilization—A review. Poult. Sci. 1993, 72, 1617–1620. [Google Scholar] [CrossRef]
- Ji, Y.; Chen, J.; Lv, J.; Li, Z.; Xing, L.; Ding, S. Extraction of keratin with ionic liquids from poultry feather. Sep. Purif. Technol. 2014, 132, 577–583. [Google Scholar] [CrossRef]
- Poole, A.J.; Church, J.S. The effects of physical and chemical treatments on Na 2 S produced feather keratin films. Int. J. Biol. Macromol. 2015, 73, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, A.; Arimoto, M.; Takeuchi, K.; Fujii, T. A Rapid Extraction Procedure of Human Hair Proteins and Identification of Phosphorylated Species. Biol. Pharm. Bull. 2002, 25, 569–572. [Google Scholar] [CrossRef] [Green Version]
- Church, J.S.; Poole, A.J.; Woodhead, A.L. The Raman analysis of films cast from dissolved feather keratin. Vib. Spectrosc. 2010, 53, 107–111. [Google Scholar] [CrossRef]
- Poole, A.J.; Lyons, R.E.; Church, J.S. Dissolving Feather Keratin Using Sodium Sulfide for Bio-Polymer Applications. J. Polym. Environ. 2011, 19, 995–1004. [Google Scholar] [CrossRef]
- Xu, H.; Ma, Z.; Yang, Y. Dissolution and regeneration of wool via controlled disintegration and disentanglement of highly crosslinked keratin. J. Mater. Sci. 2014, 49, 7513–7521. [Google Scholar] [CrossRef]
- Srinivasan, B.; Kumar, R.; Shanmugam, K.; Sivagnam, U.T.; Reddy, N.P.; Sehgal, P.K. Porous keratin scaffold–promising biomaterial for tissue engineering and drug delivery. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 92B, 5–12. [Google Scholar] [CrossRef]
- Ozaki, Y.; Takagi, Y.; Mori, H.; Hara, M. Porous hydrogel of wool keratin prepared by a novel method: An extraction with guanidine/2-mercaptoethanol solution followed by a dialysis. Mater. Sci. Eng. C 2014, 42, 146–154. [Google Scholar] [CrossRef]
- Aguayo-Villarreal, I.A.; Bonilla-Petriciolet, A.; Hernández-Montoya, V.; Montes-Morán, M.A.; Reynel-Avila, H.E. Batch and column studies of Zn2+ removal from aqueous solution using chicken feathers as sorbents. Chem. Eng. J. 2011, 167, 67–76. [Google Scholar] [CrossRef]
- Brenner, M.; Weichold, O. Protein Hydrolysates from Biogenic Waste as an Ecological Flame Retarder and Binder for Fiberboards. ACS Omega 2020, 5, 32227–32233. [Google Scholar] [CrossRef]
- Arai, K.M.; Takahashi, R.; Yokote, Y.; Akahane, K. Amino-acid sequence of feather keratin from fowl. Eur. J. Biochem. 1983, 132, 501–507. [Google Scholar] [CrossRef]
- Moore, S.; Spackman, D.H.; Stein, W.H. Chromatography of amino acids on sulfonated polystyrene resins. An improved system. Anal. Chem. 1958, 30, 1185–1190. [Google Scholar] [CrossRef]
- Barth, A. Infrared spectroscopy of proteins. Biochim. Biophys. Acta (BBA) Bioenerg. 2007, 1767, 1073–1101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barth, A.; Zscherp, C. What vibrations tell about proteins. Q. Rev. Biophys. 2002, 35, 369–430. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Yu, S. Fourier Transform Infrared Spectroscopic Analysis of Protein Secondary Structures. Acta Biochim. Biophys. Sin. 2007, 39, 549–559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, J.; Rastogi, S.; Terry, A.E.; Popescu, C. Self-organization of oligopeptides obtained on dissolution of feather keratins in superheated water. Biomacromolecules 2007, 8, 800–806. [Google Scholar] [CrossRef] [PubMed]
- Pawlukojć, A.; Leciejewicz, J.; Ramirez-Cuesta, A.; Nowicka-Scheibe, J. L-cysteine: Neutron spectroscopy, Raman, IR and ab initio study. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2005, 61, 2474–2481. [Google Scholar] [CrossRef] [PubMed]
- Kogelheide, F.; Kartaschew, K.; Strack, M.; Baldus, S.; Metzler-Nolte, N.; Havenith, M.; Awakowicz, P.; Stapelmann, K.; Lackmann, J.-W. FTIR spectroscopy of cysteine as a ready-to-use method for the investigation of plasma-induced chemical modifications of macromolecules. J. Phys. D Appl. Phys. 2016, 49, 084004. [Google Scholar] [CrossRef]
- Stefan, J. Versuche über die Verdampfung. Sitzber.K. Akad. Wiss. Wien. 1873, 68, 385–423. [Google Scholar]
- Rao, G.S.; Gorin, G. Reaction of cystine with sodium sulfide in sodium hydroxide solution. J. Org. Chem. 1959, 24, 749–753. [Google Scholar] [CrossRef]
- Liu, D.K.; Chang, S. Kinetic study of the reaction between cystine and sulfide in alkaline solutions. Can. J. Chem. 1987, 65, 770–774. [Google Scholar] [CrossRef] [Green Version]
- Harrap, B.S.; Woods, E.F. Soluble derivatives of feather keratin. 2. Molecular weight and conformation. Biochem. J. 1964, 92, 19–26. [Google Scholar] [CrossRef] [Green Version]
- Schroeder, W.; Kay, L.M.; Lewis, B.; Munger, N. The amino acid composition of certain morphologically distinct parts of white turkey feathers, and of goose feather barbs and goose down. J. Am. Chem. Soc. 1955, 77, 3901–3908. [Google Scholar] [CrossRef]
- Bianco, C.L.; Akaike, T.; Ida, T.; Nagy, P.; Bogdandi, V.; Toscano, J.P.; Kumagai, Y.; Henderson, C.F.; Goddu, R.N.; Lin, J. The reaction of hydrogen sulfide with disulfides: Formation of a stable trisulfide and implications for biological systems. Br. J. Pharmacol. 2019, 176, 671–683. [Google Scholar] [CrossRef]
- Swan, J. Mechanism of alkaline degradation of cystine residues in proteins. Nature 1957, 179, 965. [Google Scholar] [CrossRef] [PubMed]
- Rombouts, I.; Lambrecht, M.A.; Carpentier, S.C.; Delcour, J.A. Identification of lanthionine and lysinoalanine in heat-treated wheat gliadin and bovine serum albumin using tandem mass spectrometry with higher-energy collisional dissociation. Amino Acids 2016, 48, 959–971. [Google Scholar] [CrossRef]
- Daft, F.S.; Coghill, R.D. The alkaline decomposition of serine. J. Biol. Chem. 1931, 90, 341–350. [Google Scholar] [CrossRef]
- Luo, Y.; Liang, J.; Zeng, G.; Chen, M.; Mo, D.; Li, G.; Zhang, D. Seed Germination Test for Toxicity Evaluation of Compost: Its Roles, Problems and Prospects. Waste Manag. (Oxford) 2018, 71, 109–114. [Google Scholar] [CrossRef]
- Hisatake, K.; Tanaka, S.; Aizawa, Y. Evaporation rate of water in a vessel. J. Appl. Phys. 1993, 73, 7395–7401. [Google Scholar] [CrossRef]







| Sample Name | Feathers a | Na2S | Na2S/Feathers | |
|---|---|---|---|---|
| g | g | g/g | mol/mol b | |
| KG5-01 | 5 | 0.5 | 0.1 | 1.8 |
| KG5-02 | 5 | 1 | 0.2 | 3.6 |
| KG5-04 | 5 | 2 | 0.4 | 7.1 |
| KG10-02 | 10 | 2 | 0.2 | 3.6 |
| Sample Name | C [wt%] | S [wt%] | S/C Ratio (×10−2) | Total Cysteine a mol/100 mol |
|---|---|---|---|---|
| feathers | 46.0 | 1.47 | 3.2 | 9.9 |
| KG5-02 dialysed | 46.7 | 2.68 | 5.7 | 9.4 |
| Keratin hydrogel water uptake 150 g/g | 47.7 | 3.26 | 6.8 | 7.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brenner, M.; Weichold, O. Autogenous Cross-Linking of Recycled Keratin from Poultry-Feather Waste to Hydrogels for Plant-Growth Media. Polymers 2021, 13, 3581. https://doi.org/10.3390/polym13203581
Brenner M, Weichold O. Autogenous Cross-Linking of Recycled Keratin from Poultry-Feather Waste to Hydrogels for Plant-Growth Media. Polymers. 2021; 13(20):3581. https://doi.org/10.3390/polym13203581
Chicago/Turabian StyleBrenner, Markus, and Oliver Weichold. 2021. "Autogenous Cross-Linking of Recycled Keratin from Poultry-Feather Waste to Hydrogels for Plant-Growth Media" Polymers 13, no. 20: 3581. https://doi.org/10.3390/polym13203581