Interaction Insight of Pullulan-Mediated Gamma-Irradiated Silver Nanoparticle Synthesis and Its Antibacterial Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
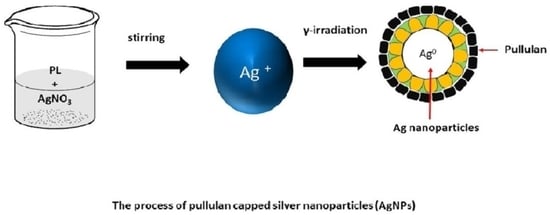
2.2.1. Synthesis of Ag-NPs on Pullulan by Using γ-Irradiation
2.2.2. Characterization Methods and Instruments
3. Results and Discussion
3.1. Synthesis and Characterization of Silver Nanoparticles from Pullulan
3.2. Formation Mechanism of Silver Nanoparticles
3.3. UV–Vis Analysis
3.4. XRD Analysis
3.5. TEM Analysis
3.6. Antimicrobial Activity of Ag-NP/PL
3.7. Antimicrobial Activity of Ag-NP/PL
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coseri, S.; Spatareanu, A.; Sacarescu, L.; Rimbu, C.M.; Suteu, D.; Spirk, S.; Harabagiu, V. Green synthesis of the silver nanoparticles mediated by pullulan and 6-carboxypullulan. Carbohydr. Polym. 2015, 116, 9–17. [Google Scholar] [CrossRef]
- Khalil, K.A.; Fouad, H.; Elsarnagawy, T.; Almajhdi, F.N. Preparation and Characterization of Electrospun PLGA/silver Composite Nanofibers for Biomedical Applications. Int. J. Electrochem. Sci. 2013, 8, 3483–3493. [Google Scholar]
- Ahmed, S.; Ahmad, M.; Swami, B.L.; Ikram, S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: A green expertise. J. Adv. Res. 2015, 7, 17–28. [Google Scholar] [CrossRef] [Green Version]
- Alishah, H.; Pourseyedi, S.; Ebrahimipour, S.Y.; Mahani, S.E.; Rafiei, N. Green synthesis of starch-mediated CuO nanoparticles: Preparation, characterization, antimicrobial activities and in vitro MTT assay against MCF-7 cell line. Rend. Lince- 2016, 28, 65–71. [Google Scholar] [CrossRef]
- Zhang, X.F.; Liu, Z.G.; Shen, W.; Gurunathan, S. Silver nanoparticles: Synthesis, characterization, properties, applications, and therapeutic approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef]
- Shameli, K.; Ahmad MBin Yunus, W.M.Z.W.; Ibrahim, N.A.; Gharayebi, Y.; Sedaghat, S. Synthesis of silver/montmorillonite nanocomposites using γ-irradiation. Int. J. Nanomed. 2010, 5, 1067–1077. [Google Scholar] [CrossRef] [Green Version]
- Kaur, A.; Preet, S.; Kumar, V.; Kumar, R.; Kumar, R. Synergetic effect of vancomycin loaded silver nanoparticles for enhanced antibacterial activity. Colloids Surf. B Biointerfaces 2018, 176, 62–69. [Google Scholar] [CrossRef]
- Sharma, V.; Yngard, R.A.; Lin, Y. Silver nanoparticles: Green synthesis and their antimicrobial activities. Adv. Colloid Interface Sci. 2009, 145, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Jayeoye, T.J.; Olatunde, O.O.; Benjakul, S.; Rujiralai, T. Synthesis and characterization of novel poly(3-aminophenyl boronic acid-co-vinyl alcohol) nanocomposite polymer stabilized silver nanoparticles with antibacterial and antioxidant applications. Colloids Surf. B Biointerfaces 2020, 193, 111112. [Google Scholar] [CrossRef] [PubMed]
- van Hengel, I.A.J.; Putra, N.E.; Tierolf, M.W.A.M.; Minneboo, M.; Fluit, A.C.; Fratila-Apachitei, L.E.; Apachitei, I.; Zadpoor, A.A. Biofunctionalization of selective laser melted porous titanium using silver and zinc nanoparticles to prevent infections by antibiotic-resistant bacteria. Acta Biomater. 2020, 107, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Briffa, S.M.; Swingler, S.; Gibson, H.; Kannappan, V.; Adamus, G.; Kowalczuk, M.M.; Martin, C.; Radecka, I. Synthesis of Silver Nanoparticles Using Curcumin-Cyclodextrins Loaded into Bacterial Cellulose-Based Hydrogels for Wound Dressing Applications. Biomacromolecules 2020, 21, 1802–1811. [Google Scholar] [CrossRef]
- Veerasamy, R.; Xin, T.Z.; Gunasagaran, S.; Xiang, T.F.W.; Yang, E.F.C.; Jeyakumar, N.; Dhanaraj, S.A. Biosynthesis of silver nanoparticles using mangosteen leaf extract and evaluation of their antimicrobial activities. J. Saudi Chem. Soc. 2011, 15, 113–120. [Google Scholar] [CrossRef] [Green Version]
- Cheng, K.C.; Demirci, A.; Catchmark, J.M. Pullulan: Biosynthesis, production, and applications. Appl. Microbiol. Biotechnol. 2011, 92, 29–44. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Ai, S.; Xie, J.; Xu, J. Comparison of direct synthesis of silver nanoparticles colloid using pullulan under conventional heating and microwave irradiation. Inorg. Nano-Met. Chem. 2017, 47, 938–945. [Google Scholar] [CrossRef]
- Akter, M.; Sikder, T.; Rahman, M.; Ullah, A.K.M.A.; Hossain, K.F.B.; Banik, S.; Hosokawa, T.; Saito, T.; Kurasaki, M. A systematic review on silver nanoparticles-induced cytotoxicity: Physicochemical properties and perspectives. J. Adv. Res. 2018, 9, 1–16. [Google Scholar] [CrossRef]
- Rahimi, M.; Noruzi, E.B.; Sheykhsaran, E.; Ebadi, B.; Kariminezhad, Z.; Molaparast, M.; Mehrabani, M.G.; Mehramouz, B.; Yousefi, M.; Ahmadi, R.; et al. Carbohydrate polymer-based silver nanocomposites: Recent progress in the antimicrobial wound dressings. Carbohydr. Polym. 2019, 231, 115696. [Google Scholar] [CrossRef]
- Mohan, S.; Oluwafemi, O.S.; Songca, S.P.; Jayachandran, V.; Rouxel, D.; Joubert, O.; Kalarikkal, N.; Thomas, S. Synthesis, antibacterial, cytotoxicity and sensing properties of starch-capped silver nanoparticles. J. Mol. Liq. 2016, 213, 75–81. [Google Scholar] [CrossRef]
- Yakout, S.M.; Mostafa, A.A. A novel green synthesis of silver nanoparticles using soluble starch and its antibacterial activity. Int. J. Clin. Exp. Med. 2015, 8, 3538–3544. [Google Scholar]
- Krklješ, A.N.; Marinović-Cincović, M.T.; Kacarevic-Popovic, Z.M.; Nedeljković, J.M. Radiolytic synthesis and characterization of Ag-PVA nanocomposites. Eur. Polym. J. 2007, 43, 2171–2176. [Google Scholar] [CrossRef]
- El-Batal, A.I.; El-Sayyad, G.; El-Ghamry, A.; Agaypi, K.; Elsayed, M.A.; Gobara, M. Melanin-gamma rays assistants for bismuth oxide nanoparticles synthesis at room temperature for enhancing antimicrobial, and photocatalytic activity. J. Photochem. Photobiol. B Biol. 2017, 173, 120–139. [Google Scholar] [CrossRef]
- Morris, M.A.; Padmanabhan, S.C.; Cruz-Romero, M.C.; Cummins, E.; Kerry, J.P. Development of active, nanoparticle, antimicrobial technologies for muscle-based packaging applications. Meat Sci. 2017, 132, 163–178. [Google Scholar] [CrossRef]
- Guan, Y. Syntheses of Novel Water-Soluble Antimicrobial Polymers and Their Application in Papermaking; The University of New Brunswick: Fredericton, NB, Canada, 2007. [Google Scholar]
- Liu, M. Synthesis of Bio-Based Nanocomposites for Controlled Release of Antimicrobial Agents in Food Packaging; Food Science; The Pennsylvania State University: State College, PA, USA, 2014. [Google Scholar]
- Mauriello, G. Control of Microbial Activity Using Antimicrobial Packaging. In Jorge Barros-Velázquez, editor. Antimicrobial Food Packaging; Academic Press, Elsevier: Amsterdam, The Netherlands, 2015; p. 141. [Google Scholar]
- Bourtoom, T.; Chinnan, M.S. Preparation and properties of rice starch–chitosan blend biodegradable film. LWT 2008, 41, 1633–1641. [Google Scholar] [CrossRef]
- Elsabee, M.Z.; Abdou, E.S. Chitosan based edible films and coatings: A review. Mater. Sci. Eng. C 2013, 33, 1819–1841. [Google Scholar] [CrossRef] [PubMed]
- Cheviron, P.; Gouanvé, F.; Espuche, E. Green synthesis of colloid silver nanoparticles and resulting biodegradable starch/silver nanocomposites. Carbohydr. Polym. 2014, 108, 291–298. [Google Scholar] [CrossRef]
- Salari, Z.; Danafar, F.; Dabaghi, S.; Ataei, S.A. Sustainable synthesis of silver nanoparticles using macroalgae Spirogyra varians and analysis of their antibacterial activity Sustainable synthesis of silver nanoparticles using macroalgae Spirogyra varians. J. Saudi Chem. Soc. 2016, 20, 459–464. [Google Scholar] [CrossRef] [Green Version]
- Rao, Y.; Banerjee, D.; Datta, A.; Das, S.; Guin, R.; Saha, A. Gamma irradiation route to synthesis of highly re-dispersible natural polymer capped silver nanoparticles. Radiat. Phys. Chem. 2010, 79, 1240–1246. [Google Scholar] [CrossRef]
- Ghazy, O.A.; Nabih, S.; Abdel-Moneam, Y.K.; Senna, M.M. Synthesis and characterization of silver/zein nanocomposites and their application. Polym. Compos. 2017, 38, E9–E15. [Google Scholar]
- Kumar, B.; Smita, K.; Cumbal, L.; Debut, A. Green synthesis of silver nanoparticles using Andean blackberry fruit extract. Saudi J. Biol. Sci. 2015, 24, 45–50. [Google Scholar] [CrossRef] [Green Version]
- Desai, K.; Kit, K.; Li, J.; Zivanovic, S. Morphological and Surface Properties of Electrospun Chitosan Nanofibers. Biomacromolecules 2008, 9, 1000–1006. [Google Scholar] [CrossRef]
- Chen, P.; Song, L.; Liu, Y.; Fang, Y.-E. Synthesis of silver nanoparticles by γ-ray irradiation in acetic water solution containing chitosan. Radiat. Phys. Chem. 2007, 76, 1165–1168. [Google Scholar] [CrossRef]
- Sheikh, N.; Akhavan, A.; Kassaee, M. Synthesis of antibacterial silver nanoparticles by γ-irradiation. Phys. E Low-Dimens. Syst. Nanostructures 2009, 42, 132–135. [Google Scholar] [CrossRef]
- Janata, E. Structure of the Trimer Silver Cluster Ag 32+ †. J. Phys. Chem. B 2003, 109, 7334–7336. [Google Scholar] [CrossRef]
- Janata, E.; Henglein, A.; Ershov, B.G. First Clusters of Ag+ Ion Reduction in Aqueous Solution. J. Phys. Chem. 1994, 98, 10888–10890. [Google Scholar] [CrossRef]
- Goia D., V. Preparation and formation mechanisms of uniform metallic particles in homogeneous solutions. J. Mater. Chem. 2004, 14, 451–458. [Google Scholar] [CrossRef]
- Hosny, A.M.S.; Kashef, M.T.; Rasmy, S.A.; Aboul-Magd, D.S.; El-Bazza, Z.E. Antimicrobial activity of silver nanoparticles synthesized using honey and gamma radiation against silver-resistant bacteria from wounds and burns. Adv. Nat. Sci. Nanosci. Nanotechnol. 2017, 8, 045009. [Google Scholar] [CrossRef] [Green Version]
- Razo-Lazcano, T.A.; Solans, C.; González-Muñoz, M.P.; Rivera-Rangel, R.D.; Avila-Rodriguez, M. Green synthesis of silver nanoparticles in oil-in-water microemulsion and nano-emulsion using geranium leaf aqueous extract as a reducing agent. Colloids Surf. A Phys. Eng Asp 2017, 536, 60–67. [Google Scholar] [CrossRef]
- Biliaderis, C.; Lazaridou, A.; Mavropoulos, A.; Barbayiannis, N. WATER PLASTICIZATION EFFECTS ON CRYSTALLIZATION BEHAVIOR OF LACTOSE IN A CO-LYOPHILIZED AMORPHOUS POLYSACCHARIDE MATRIX AND ITS RELEVANCE TO THE GLASS TRANSITION. Int. J. Food Prop. 2002, 5, 463–482. [Google Scholar] [CrossRef]
- Sun, J.-T.; Li, J.-W.; Tsou, C.-H.; Pang, J.-C.; Chung, R.-J.; Chiu, C.-W. Polyurethane/Nanosilver-Doped Halloysite Nanocomposites: Thermal, Mechanical Properties, and Antibacterial Properties. Polymers 2020, 12, 2729. [Google Scholar] [CrossRef]
- Afify, T.; Saleh, H.; Ali, Z. Structural and morphological study of gamma-irradiation synthesized silver nanoparticles. Polym. Compos. 2015, 38, 2687–2694. [Google Scholar] [CrossRef]
- Yoksan, R.; Chirachanchai, S. Silver nanoparticles dispersing in chitosan solution: Preparation by γ-ray irradiation and their antimicrobial activities. Mater. Chem. Phys. 2009, 115, 296–302. [Google Scholar] [CrossRef]
- Yoksan, R.; Chirachanchai, S. Silver nanoparticle-loaded chitosan-starch based films: Fabrication and evaluation of tensile, barrier and antimicrobial properties. Mater. Sci. Eng. C 2010. [CrossRef]
- Patil, M.; Singh, R.; Koli, P.; Patil, K.T.; Jagdale, B.S.; Tipare, A.R.; Kim, G.-D. Antibacterial potential of silver nanoparticles synthesized using Madhuca longifolia flower extract as a green resource. Microb. Pathog. 2018, 121, 184–189. [Google Scholar] [CrossRef]
- Price, M.; Reiners, J.J.; Santiago, A.M.; Kessel, D. Monitoring Singlet Oxygen and Hydroxyl Radical Formation with Fluorescent Probes During Photodynamic Therapy. Photochem. Photobiol. 2009, 85, 1177–1181. [Google Scholar] [CrossRef] [Green Version]
- Wei, D.; Sun, W.; Qian, W.; Ye, Y.; Ma, X. The synthesis of chitosan-based silver nanoparticles and their antibacterial activity. Carbohydr. Res. 2009, 344, 2375–2382. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Du, Y.; Fan, L.; Liu, H.; Hu, Y. Chitosan-metal complexes as antimicrobial agent: Synthesis, characterization and Structure-activity study. Polym. Bull. 2005, 55, 105–113. [Google Scholar] [CrossRef]
- Qing, Y.; Cheng, L.; Li, R.; Liu, G.; Zhang, Y.; Tang, X.; Wang, J.; Liu, H.; Qin, Y. Potential antibacterial mechanism of silver nanoparticles and the optimization of orthopedic implants by advanced modification technologies. Int. J. Nanomed. 2018, 13, 3311–3327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, X.; He, B.; Liu, L.; Qu, G.; Shi, J.; Hu, L.; Jiang, G. Antibacterial mechanism of silver nanoparticles in Pseudomonas aeruginosa: Proteomics approach. Metallomics 2018, 10, 557–564. [Google Scholar] [CrossRef]
- Liau, S.Y.; Read, D.C.; Pugh, W.J.; Furr, J.R.; Russell, A.D. Interaction of silver nitrate with readily identifiable groups: Relationship to the antibacterial action of silver ions. Lett. Appl. Microbiol. 1997. [CrossRef] [PubMed]












| Sample | Average Diameter of Inhibition Zone (mm) |
|---|---|
| 0 kGy | 0 ± 0 |
| 10 kGy | 10 ± 1.65 |
| 25 kGy | 11 ± 2.34 |
| 50 kGy | 13 ± 2.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salleh, M.S.N.; Ali, R.R.; Shameli, K.; Hamzah, M.Y.; Kasmani, R.M.; Nasef, M.M. Interaction Insight of Pullulan-Mediated Gamma-Irradiated Silver Nanoparticle Synthesis and Its Antibacterial Activity. Polymers 2021, 13, 3578. https://doi.org/10.3390/polym13203578
Salleh MSN, Ali RR, Shameli K, Hamzah MY, Kasmani RM, Nasef MM. Interaction Insight of Pullulan-Mediated Gamma-Irradiated Silver Nanoparticle Synthesis and Its Antibacterial Activity. Polymers. 2021; 13(20):3578. https://doi.org/10.3390/polym13203578
Chicago/Turabian StyleSalleh, Mohd Shahrul Nizam, Roshafima Rasit Ali, Kamyar Shameli, Mohd Yusof Hamzah, Rafiziana Md Kasmani, and Mohamed Mahmoud Nasef. 2021. "Interaction Insight of Pullulan-Mediated Gamma-Irradiated Silver Nanoparticle Synthesis and Its Antibacterial Activity" Polymers 13, no. 20: 3578. https://doi.org/10.3390/polym13203578