Comparative Characterization of Hot-Pressed Polyamide 11 and 12: Mechanical, Thermal and Durability Properties
Abstract
:1. Introduction
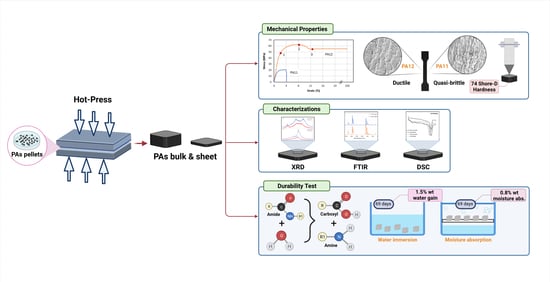
2. Materials and Methods
2.1. Materials and Sample Preparation
2.2. Mechanical Test
2.3. X-ray Diffraction
2.4. Differential Scanning Calorimetric (DSC) Technique
2.5. Fourier Transform Infrared Spectroscopy (FTIR)
2.6. Durability Test
2.7. Hardness Measurement
3. Results and Discussion
3.1. Tensile Strength
3.2. X-ray Diffraction
3.3. DSC Analyses
3.4. FTIR-ATR Analyses
3.5. Durability Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Deopura, B.; Alagirusamy, R.; Joshi, M.; Gupta, B. Polyesters and Polyamides; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- MacGregor, A.; Graham, J.; Stafford, G.; Huggett, R. Recent experiences with denture polymers. J. Dent. 1984, 12, 146–157. [Google Scholar] [CrossRef]
- Rahim, T.; Abdullah, A.; Akil, H.M.; Mohamad, D.; Rajion, Z. The improvement of mechanical and thermal properties of pol-yamide 12 3D printed parts by fused deposition modelling. Express Polym. Lett. 2017, 11, 963–982. [Google Scholar] [CrossRef]
- Primc, G. Surface modification of polyamides by gaseous plasma—Review and scientific challenges. Polymer 2020, 12, 3020. [Google Scholar] [CrossRef] [PubMed]
- Štěpánová, V.; Šrámková, P.; Sihelník, S.; Stupavská, M.; Jurmanová, J.; Kováčik, D. The effect of ambient air plasma generated by coplanar and volume dielectric barrier discharge on the surface characteristics of polyamide foils. Vacuum 2021, 183, 109887. [Google Scholar] [CrossRef]
- Martino, L.; Basilissi, L.; Farina, H.; Ortenzi, M.A.; Zini, E.; di Silvestro, G.; Scandola, M. Bio-based polyamide 11: Synthesis, rhe-ology and solid-state properties of star structures. Eur. Polym. J. 2014, 59, 69–77. [Google Scholar] [CrossRef]
- Lao, S.; Yong, W.; Nguyen, K.; Moon, T.; Koo, J.; Pilato, L.; Wissler, G. Flame-retardant polyamide 11 and 12 nanocomposites: Processing, morphology, and mechanical properties. J. Compos. Mater. 2010, 44, 2933–2951. [Google Scholar] [CrossRef]
- Jia, N.; Kagan, V.A. Interpretations of tensile properties of polyamide 6 and PET based thermoplastics using ASTM and ISO procedures. In Limitations of Test Methods for Plastics; ASTM International: Pennsylvania, PA, USA, 2008; p. 54. [Google Scholar]
- Marchildon, K. Polyamides—Still Strong After Seventy Years. Macromol. React. Eng. 2011, 5, 22–54. [Google Scholar] [CrossRef]
- Chemsystems Online. PERP Program—Nylon 6 and Nylon 66; White Plains Inc.: White Plains, NY, USA, 2009. [Google Scholar]
- Telen, L.; van Puyvelde, P.; Goderis, B. Random copolymers from polyamide 11 and polyamide 12 by reactive extrusion: Syn-thesis, eutectic phase behavior, and polymorphism. Macromolecules 2016, 49, 876–890. [Google Scholar] [CrossRef]
- Zhou, C.; Qi, S.; Zhu, P.; Zhao, Y.; Xu, Y.; Dong, X.; Wang, D. The methylene infrared vibration and dielectric behavior monitored by amide group arrangement for long chain polyamides. Polymer 2020, 190, 122231. [Google Scholar] [CrossRef]
- Salazar, A.; Rico, A.; Rodríguez, J.; Escudero, J.S.; Seltzer, R.; de la Escalera Cutillas, F.M. Monotonic loading and fatigue response of a bio-based polyamide PA11 and a petrol-based polyamide PA12 manufactured by selective laser sintering. Eur. Polym. J. 2014, 59, 36–45. [Google Scholar] [CrossRef]
- Griehl, W.; Ruestem, D. Nylon-12-preparation, properties, and applications. Ind. Eng. Chem. 1970, 62, 16–22. [Google Scholar] [CrossRef]
- Hiramatsu, N.; Haraguchi, K.; Hirakawa, S. Study of transformations among α, γ and γ’forms in nylon 12 by x-ray and DSC. Jpn. J. Appl. Phys. 1983, 22, 335. [Google Scholar] [CrossRef]
- Wu, J.; Xu, X.; Zhao, Z.; Wang, M.; Zhang, J. Study in performance and morphology of polyamide 12 produced by selective laser sintering technology. Rapid Prototyp. J. 2018, 24, 813–820. [Google Scholar] [CrossRef]
- Li, L.; Koch, M.H.; de Jeu, W.H. Crystalline structure and morphology in nylon-12: A small-and wide-angle X-ray scattering study. Macromolecules 2003, 36, 1626–1632. [Google Scholar] [CrossRef]
- Page, I. Polyamides as Engineering Thermoplastic Materials; iSmithers Rapra Publishing: Shrewsbury, Shropshire, UK, 2000. [Google Scholar]
- Ullah, H.; Harland, A.R.; Silberschmidt, V.V. Dynamic bending behaviour of woven composites for sports products: Experiments and damage analysis. Mater. Des. 2015, 88, 149–156. [Google Scholar] [CrossRef] [Green Version]
- Pelt, M.V. Bio-Based Polyamides: Environmental Impact and Applicability in Soccer Shoe Outsoles. Master’s Thesis, Utrecht University, Utrecht, The Netherlands, 2016. [Google Scholar]
- Williams, G.; Trask, R.; Bond, I. A self-healing carbon fibre reinforced polymer for aerospace applications. Compos. Part A Appl. Sci. Manuf. 2007, 38, 1525–1532. [Google Scholar] [CrossRef]
- Paggi, R.; Salmoria, G.; Lago, A.; Beal, V. Functionally graded PA12/MWCNTs composite fabricated by SLS to aerospace ap-plications: Mechanical and electrical behavior. In Innovative Developments in Design and Manufacturing; CRC Press: Boca Raton, FL, USA, 2009; pp. 241–246. [Google Scholar]
- Griffiths, K. Polyamides in aerospace industry. Trans. IMF 2007, 85, 235–236. [Google Scholar] [CrossRef]
- Mayyas, A.; Qattawi, A.; Omar, M.; Shan, D. Design for sustainability in automotive industry: A comprehensive review. Renew. Sustain. Energy Rev. 2012, 16, 1845–1862. [Google Scholar] [CrossRef]
- Iwasa, T.; Funaki, A.; Nishi, E.; Laganis, E.; Isobe, N.; Nishioka, T.; Nakamura, K. Development of Two-Layer Fuel Tube System Consisting of Adhesive-ETFE And Modified-PA12; SAE International: Warrendale, PA, USA, 2004. [Google Scholar]
- Bhattacharya, A.B.; Chatterjee, T.; Naskar, K. Automotive applications of thermoplastic vulcanizates. J. Appl. Polym. Sci. 2020, 137, 49181. [Google Scholar] [CrossRef]
- Heimrich, M.; Bönsch, M.; Nickl, H.; Simat, T. Cyclic oligomers in polyamide for food contact material: Quantification by HPLC-CLND and single-substance calibration. Food Addit. Contam. Part A 2012, 29, 846–860. [Google Scholar] [CrossRef]
- Stoffers, N.H.; Dekker, M.; Linssen, J.P.H.; Störmer, A.; Franz, R. Alternative fatty food simulants and diffusion kinetics of nylon 12 food packaging. Food Addit. Contam. 2003, 20, 949–959. [Google Scholar] [CrossRef]
- Gill, Y.Q.; Abid, U.; Song, M. High performance Nylon12/clay nanocomposites for potential packaging applications. J. Appl. Polym. Sci. 2020, 137, 49247. [Google Scholar] [CrossRef]
- Campbell, M.; Bureau, M.N. Performance of CF/PA12 composite femoral stems. J. Mater. Sci. Mater. Med. 2007, 19, 683–693. [Google Scholar] [CrossRef]
- Rahim, E. Nondestructive Evaluation of a Polymer Composite Hip Implant Using Lock-In Thermography. Master’s Thesis, Ryerson University, Ontario, ON, Canada, 2010. [Google Scholar]
- Rahim, T.N.A.T.; Abdullah, A.M.; Akil, H.M.; Mohamad, D. Comparison of mechanical properties for polyamide 12 compo-site-based biomaterials fabricated by fused filament fabrication and injection molding. AIP Conf. Proc. 2016, 1791, 020007. [Google Scholar]
- Mason, J.F.; Stanley, M.; Ponda, A.; Demicoli, D. Case study: Engineered polyamide 12 (PA12) pipeline liner for management of sour gas corrosion at elevated temperatures. In CORROSION 2017; OnePetro: Richardson, TX, USA, 2017. [Google Scholar]
- Khalid, H.U.; Ismail, M.C.; Nosbi, N. Permeation damage of polymer liner in oil and gas pipelines: A review. Polymer 2020, 12, 2307. [Google Scholar] [CrossRef]
- Blevins, C.J. Characterization of Polyamide-12; Hydrolysis Kinetics Comparison, and the Study of Crude-oil Pipeline Appli-cations. Bachelor’s Thesis, College of William and Mary, Williamsburg, VA, USA, 2013. [Google Scholar]
- Panaitescu, D.M.; Gabor, R.A.; Frone, A.N.; Vasile, E. Influence of thermal treatment on mechanical and morphological char-acteristics of polyamide 11/cellulose nanofiber nanocomposites. J. Nanomater. 2015, 16, 4. [Google Scholar]
- McKeen, L.W. Fatigue and Tribological Properties of Plastics and Elastomers; William Andrew Publishing: Norwich, NY, USA, 2016. [Google Scholar]
- Jacques, B.; Werth, M.; Merdas, I.; Thominette, F.; Verdu, J. Hydrolytic ageing of polyamide 11. 1. Hydrolysis kinetics in water. Polymer 2002, 43, 6439–6447. [Google Scholar] [CrossRef]
- Nayak, P.L. Natural Oil-Based Polymers: Opportunities and challenges. J. Macromol. Sci. Part C 2000, 40, 1–21. [Google Scholar] [CrossRef]
- Zhang, G.; Li, Y.; Yan, D. Polymorphism in nylon-11/montmorillonite nanocomposite. J. Polym. Sci. Part B Polym. Phys. 2003, 42, 253–259. [Google Scholar] [CrossRef]
- Mathias, L.J.; Powell, D.G.; Autran, J.P.; Porter, R.S. Nitrogen-15 NMR characterization of multiple crystal forms and phase transitions in polyundecanamide (Nylon 11). Macromolecules 1990, 23, 963–967. [Google Scholar] [CrossRef]
- Nair, S.S.; Ramesh, C.; Tashiro, K. Crystalline phases in nylon-11: Studies using HTWAXS and HTFTIR. Macromolecules 2006, 39, 2841–2848. [Google Scholar] [CrossRef]
- Botelho, E.; Rezende, M.; Lauke, B. Mechanical behavior of carbon fiber reinforced polyamide composites. Compos. Sci. Technol. 2003, 63, 1843–1855. [Google Scholar] [CrossRef]
- Kuciel, S.; Kuzniar, P.; Liber-Knec, A. Polyamides from renewable sources as matrices of short fiber reinforced biocomposites. Polimer 2012, 57, 627–634. [Google Scholar] [CrossRef]
- Feldmann, M.; Bledzki, A.K. Bio-based polyamides reinforced with cellulosic fibres—Processing and properties. Compos. Sci. Technol. 2014, 100, 113–120. [Google Scholar] [CrossRef]
- Oksman, K.; Mathew, A.P.; Sain, M. Novel bionanocomposites: Processing, properties and potential applications. Plast. Rubber Compos. 2009, 38, 396–405. [Google Scholar] [CrossRef]
- Bahrami, M.; Abenojar, J.; Martínez, M.Á. Recent progress in hybrid biocomposites: Mechanical properties, water absorption, and flame retardancy. Materials 2020, 13, 5145. [Google Scholar] [CrossRef]
- Cai, C.; Tey, W.S.; Chen, J.; Zhu, W.; Liu, X.; Liu, T.; Zhao, L.; Zhou, K. Comparative study on 3D printing of polyamide 12 by selective laser sintering and multi jet fusion. J. Mater. Process. Technol. 2021, 288, 116882. [Google Scholar] [CrossRef]
- Lanzl, L.; Wudy, K.; Greiner, S.; Drummer, D. Selective laser sintering of copper filled polyamide 12: Characterization of powder properties and process behavior. Polym. Compos. 2018, 40, 1801–1809. [Google Scholar] [CrossRef]
- Pandelidi, C.; Lee, K.P.M.; Kajtaz, M. Effects of polyamide-11 powder refresh ratios in multi-jet fusion: A comparison of new and used powder. Addit. Manuf. 2021, 40, 101933. [Google Scholar]
- Jariyavidyanont, K.; Williams, J.L.; Rhoades, A.M.; Kühnert, I.; Focke, W.; Androsch, R. Crystallization of polyamide 11 during injection molding. Polym. Eng. Sci. 2017, 58, 1053–1061. [Google Scholar] [CrossRef]
- Meyer, T.; Harland, A.; Haworth, B.; Holmes, C.; Lucas, T.; Sherratt, P. The influence of different melt temperatures on the me-chanical properties of injection molded PA-12 and the post process detection by thermal analysis. Int. Polym. Process. 2017, 32, 90–101. [Google Scholar] [CrossRef]
- Yang, X.; Yang, W.; Fan, J.; Wu, J.; Zhang, K. Effects of molding on property of thermally conductive and electrically insulating polyamide 6–based composite. J. Thermoplast. Compos. Mater. 2019, 32, 1190–1203. [Google Scholar] [CrossRef]
- Campoy, I.; Gómez, M.; Marco, C. Structure and thermal properties of blends of nylon 6 and a liquid crystal copolyester. Polymer 1998, 39, 6279–6288. [Google Scholar] [CrossRef]
- Wunderlich, B. Thermal Analysis of Polymeric Materials; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2005. [Google Scholar]
- Kaiser, G.; Füglein, E.; Jeschke, Y.; Kapsch, E.; Steidl, D. Handbook Differential Scanning Calorimetry (DSC) on Polymers; NETZSCH-Gerätebau GmbH: Selb, Germany, 2015. [Google Scholar]
- Acierno, S.; Van Puyvelde, P. Rheological behavior of polyamide 11 with varying initial moisture content. J. Appl. Polym. Sci. 2005, 97, 666–670. [Google Scholar] [CrossRef]
- Nguyen, P.H.; Spoljaric, S.; Seppälä, J. Redefining polyamide property profiles via renewable long-chain aliphatic segments: Towards impact resistance and low water absorption. Eur. Polym. J. 2018, 109, 16–25. [Google Scholar] [CrossRef]
- Meyer, A.; Jones, N.; Lin, Y.; Kranbuehl, D. Characterizing and modeling the hydrolysis of polyamide-11 in a pH 7 water en-vironment. Macromolecules 2002, 35, 2784–2798. [Google Scholar] [CrossRef]
- Paolucci, F.; Peters, G.W.; Govaert, L.E. Plasticity-controlled failure of sintered and molded polyamide 12: Influence of temper-ature and water absorption. J. Appl. Polym. Sci. 2020, 137, 48525. [Google Scholar] [CrossRef] [Green Version]
- Bélec, L. Contribution of the amorphous and crystal phase of polyamide 11 and polyamide 12 during their straining in the transition range. Doctoral dissertation. Poitiers 1995. [Google Scholar]
- Maïza, S.; Lefebvre, X.; Brusselle-Dupend, N.; Klopffer, M.H.; Cangémi, L.; Castagnet, S.; Grandidier, J.C. Physicochemical and mechanical degradation of polyamide 11 induced by hydrolysis and thermal aging. J. Appl. Polym. Sci. 2019, 136, 47628. [Google Scholar] [CrossRef]
- Wietzke, S.; Jansen, C.; Reuter, M.; Jung, T.; Hehl, J.; Kraft, D.; Chatterjee, S.; Greiner, A.; Koch, M. Thermomorphological study of the terahertz lattice modes in polyvinylidene fluoride and high-density polyethylene. Appl. Phys. Lett. 2010, 97, 022901. [Google Scholar] [CrossRef]
- Kausch, H.-H.; Gensler, R.; Grein, C.; Plummer, C.J.G.; Scaramuzzino, P. Crazing in semicrystalline thermoplastics. J. Macromol. Sci. Part B 1999, 38, 803–815. [Google Scholar] [CrossRef]
- Zhang, Y.; Ben Jar, P.-Y.; Xue, S.; Li, L. Quantification of strain-induced damage in semi-crystalline polymers: A review. J. Mater. Sci. 2018, 54, 62–82. [Google Scholar] [CrossRef]
- Gümüs, S.; Lackner, J.M.; Polat, Şeyda; Kraschitzer, W.; Hanning, H.; Bayram, A.; Kaya, M.; Çallı, M.; Alkan, A. Failure behavior of PA12 based SLS lattice structure with macro-porosity. In MATEC Web of Conferences; EDP Sciences: Les Ulis, France, 2018; Volume 188, p. 03007. [Google Scholar]
- Kinloch, A.J.; Young, R.J. Fracture Behaviour of Polymers; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1995. [Google Scholar]
- Russo, P.; Simeoli, G.; Vitiello, L.; Filippone, G. Bio-Polyamide 11 Hybrid Composites Reinforced with Basalt/Flax Interwoven Fibers: A Tough Green Composite for Semi-Structural Applications. Fibers 2019, 7, 41. [Google Scholar] [CrossRef] [Green Version]
- Huynh, M.D.; Trung, T.H.; Van Cong, D.; Hoang, T.; Dantras, E.; Lacabanne, C.; Giang, N.V. Effect of Maleic Anhydride Grafted Ethylene Vinyl Acetate Compatibilizer on the Mechanical, Thermal Properties and Weathering Resistance of Polyamide 11/Bamboo Fiber Composite. Mater. Trans. 2020, 61, 1527–1534. [Google Scholar] [CrossRef]
- Takahashi, Y.; Suzumoto, Y.; Enomoto, K. Functionalization of carbon nanofiber and its effect on tensile properties of PA11 matrix composite reinforced with carbon nanofiber. AIP Conf. Proc. 2016, 1779, 040001. [Google Scholar]
- Francisco, D.L.; De Paiva, L.B.; Aldeia, W.; Lugão, A.B.; Moura, E.A.B. Investigation on mechanical behaviors of polyamide 11 reinforced with halloysite nanotubes. In Characterization of Minerals, Metals, and Materials; Springer: Cham, Switzerland, 2019; pp. 693–701. [Google Scholar]
- Zierdt, P.; Theumer, T.; Kulkarni, G.; Däumlich, V.; Klehm, J.; Hirsch, U.; Weber, A. Sustainable wood-plastic composites from bio-based polyamide 11 and chemically modified beech fibers. Sustain. Mater. Technol. 2015, 6, 6–14. [Google Scholar] [CrossRef]
- Walter, R.; Friedrich, K.; Gurka, M. Characterization of mechanical properties of additively manufactured polymers and composites. In AIP Conference Proceedings; AIP Publishing: College Park, MD, USA, 2018; Volume 1981, p. 020033. [Google Scholar]
- Almansoori, A.; Majewski, C.; Rodenburg, C. Nanoclay/polymer composite powders for use in laser sintering applications: Effects of nanoclay plasma treatment. JOM 2017, 69, 2278–2285. [Google Scholar] [CrossRef] [Green Version]
- Connor, H.J.O.; Dowling, D.P. Comparison between the properties of polyamide 12 and glass bead filled polyamide 12 using the multi jet fusion printing process. Addit. Manuf. 2020, 31, 100961. [Google Scholar] [CrossRef]
- Doagou-Rad, S.; Islam, A.; Antusch, S.; Jung, J.; Klein, A.; Plewa, K.; Piotter, V. Investigation of conductive hybrid polymer composites reinforced with copper micro fibers and carbon nanotubes produced by injection molding. Mater. Today Commun. 2019, 20, 100566. [Google Scholar] [CrossRef]
- Aharoni, S.M. n-Nylons: Their Synthesis, Structure, and Properties; Wiley-Blackwell: Hoboken, NJ, USA, 1997. [Google Scholar]
- Fornes, T.; Paul, D. Structure and properties of nanocomposites based on nylon-11 and-12 compared with those based on ny-lon-6. Macromolecules 2004, 37, 7698–7709. [Google Scholar] [CrossRef]
- Hu, G.S.; Ding, Z.Y.; Li, Y.C.; Wang, B.B. Crystalline morphology and melting behavior of nylon11/ethylene-vinyl alco-hol/dicumyl peroxide blends. J. Polym. Res. 2009, 16, 263–269. [Google Scholar] [CrossRef]
- Liu, T.; Lim, K.P.; Tjiu, W.C.; Pramoda, K.; Chen, Z.-K. Preparation and characterization of nylon 11/organoclay nanocomposites. Polymer 2003, 44, 3529–3535. [Google Scholar] [CrossRef]
- Liu, T.; Chen, D.; Phang, I.Y.; Wei, C. Studies on crystal transition of polyamide 11 nanocomposites by variable-temperature X-ray diffraction. Chin. J. Polym. Sci. 2013, 32, 115–122. [Google Scholar] [CrossRef]
- Ricou, P.; Pinel, E.; Juhasz, N. Temperature experiments for improved accuracy in the calculation of polyamide-11 crystallinity by X-ray diffraction. Adv. X-ray Anal. 2005, 48, 171–175. [Google Scholar]
- Bai, L.; Hong, Z.; Wang, D.; Li, J.; Wang, X.; Pan, G.; Li, L.; Li, X. Deformation-induced phase transitions of polyamide 12 in its elastomer segmented copolymers. Polymer 2010, 51, 5604–5611. [Google Scholar] [CrossRef]
- Inoue, K.; Hoshino, S. Crystal structure of nylon 12. J. Polym. Sci. Polym. Phys. Ed. 1973, 11, 1077–1089. [Google Scholar] [CrossRef]
- Quarti, C.; Milani, A.; Civalleri, B.; Orlando, R.; Castiglioni, C. Ab initio calculation of the crystalline structure and IR spectrum of polymers: Nylon 6 polymorphs. J. Phys. Chem. B 2012, 116, 8299–8311. [Google Scholar] [CrossRef]
- Arabnejad, S.; Manzhos, S.; He, C.; Shim, V.P.W. Shear-induced conformation change in α-crystalline nylon6. Appl. Phys. Lett. 2014, 105, 221910. [Google Scholar] [CrossRef]
- Bernadó, P.; Alemán, C.; Puiggalí, J. Relative stability between the α and γ forms of even nylons based on group contributions. Eur. Polym. J. 1999, 35, 835–847. [Google Scholar] [CrossRef]
- Dasgupta, S.; Hammond, W.B.; Goddard, W.A. Crystal structures and properties of nylon polymers from theory. J. Am. Chem. Soc. 1996, 118, 12291–12301. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts; Wiley: Hoboken, NJ, USA, 2004. [Google Scholar]
- Seltzer, R.; de la Escalera, F.M.; Segurado, J. Effect of water conditioning on the fracture behavior of PA12 composites processed by selective laser sintering. Mater. Sci. Eng. A 2011, 528, 6927–6933. [Google Scholar] [CrossRef]
- McKeen, L.W. The Effect of Creep and Other Time Related Factors on Plastics and Elastomers; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Tanaka, K.; Mizuno, S.; Honda, H.; Katayama, T.; Enoki, S. Effect of water absorption on the mechanical properties of carbon fiber/polyamide composites. J. Solid Mech. Mater. Eng. 2013, 7, 520–529. [Google Scholar] [CrossRef] [Green Version]
- Mazan, T.; Berggren, R.; Jørgensen, J.K.; Echtermeyer, A. Aging of polyamide 11. Part 1: Evaluating degradation by thermal, mechanical, and viscometric analysis. J. Appl. Polym. Sci. 2015, 132, 20. [Google Scholar] [CrossRef]
- Roy, S. Moisture-induced degradation. In Long-Term Durability of Polymeric Matrix Composites; Springer: Boston, MA, USA, 2012; pp. 181–236. [Google Scholar]
- Venoor, V.; Park, J.H.; O Kazmer, D.; Sobkowicz, M.J. Understanding the effect of water in polyamides: A review. Polym. Rev. 2021, 61, 598–645. [Google Scholar] [CrossRef]
- El-Mazry, C.; Correc, O.; Colin, X. A new kinetic model for predicting polyamide 6-6 hydrolysis and its mechanical embrittle-ment. Polym. Degrad. Stab. 2012, 97, 1049–1059. [Google Scholar] [CrossRef] [Green Version]
- Caglieri, S.C.; Pagnan, M. Theoretical study on the acid hydrolysis of aliphatic and aromatic amides. Inf. tecnológica 2013, 24, 35–40. [Google Scholar] [CrossRef]
- Smith, P. The Effect of Ethanol, Methanol, and Water on the Hydrolytic Degradation of Polyamide-11. Bachelor’s Thesis, College of William and Mary, Williamsburg, VA, USA, 2016. [Google Scholar]


















| E (MPa) | Ultimate Strength (MPa) | Elongation at Break (%) | |
|---|---|---|---|
| PA11 | 1762.2 ± 138.3 | 20.0 ± 3.8 | 4 |
| PA12 | 2964.3 ± 102.8 | 64.0 ± 3.2 | 200 |
| Manufacturing Technique | E (GPa) | Ultimate Strength (MPa) | Elongation at Break (%) | χc (%) | Ref. | |
|---|---|---|---|---|---|---|
| PA11 | Hot press | 1.7 | 20 | 4 | 23 | Present work |
| Hot press | 1.0 | 44.9 | 26 | 27 | [68] | |
| Hot press | 0.3 | 32 | 252 | - | [69] | |
| SLS | 1.3 | 49.6 | - | 28 | [7] | |
| Injection molding | - | 39 | Without break | - | [70] | |
| Extrusion | 1.2 | 36.5 | 235 | - | [71] | |
| Injection molding | 1.3 | 48 | 137 | - | [72] | |
| PA12 | Hot press | 2.9 | 64 | 200 | 49 | Present work |
| Hot press | 1.7 | 51.2 | 163 | [73] | ||
| Hot press | 0.8 | 39.6 | 24 | - | [74] | |
| HP jet fusion | 1.2 | 47 | 19 | 26 | [75] | |
| Injection molding | 1.3 | 35 | 230 | - | [76] | |
| SLS | 1.7 | 42.2 | 7 | - | [73] | |
| SLS | 1.4 | 33 | - | - | [7] |
| Phase | 2θ° | |
|---|---|---|
| PA11 | α (triclinic) | 20.5 |
| 23.7 | ||
| PA12 | γ (hexagonal) | 21.45 |
| PA11 | PA12 | ||||||
|---|---|---|---|---|---|---|---|
| 1st Heating | Cooling | 2nd Heating | 1st Heating | Cooling | 2nd Heating | ||
| Tg (°C) | 44.49 | 45.95 | Tg (°C) | 42.77 | 49.29 | ||
| Tm (°C) | 193.90 | 188.50 | Tm (°C) | 178.86 | 178.05 | ||
| Tc (°C) | 162.52 | Tc (°C) | 144.07 | ||||
| ∆H (J/g) | 51.87 | 39.27 | 47.86 | ∆H (J/g) | 46.52 | 57.15 | 47.15 |
| χc (%) | 23.16 | 17.53 | 21.37 | χc (%) | 49.48 | 60.16 | 49.63 |
| Frequency (cm−1) | Vibration |
|---|---|
| 3301 | N−H stretching strong band/OH |
| 3082 | NH groups weak band |
| 2918 | CH2 asymmetric stretching |
| 2850 | CH2 symmetric stretching |
| 1732 | O−C=O |
| 1635 | Amide I, C=O stretching |
| 1550 | Amide II, C- stretching + C=O in-plane bending |
| 1465 | CH2 bending asym |
| 1367 | CH2 bending sym |
| 1275 | Amide III, NH−O stretching |
| 1226 | C−O−C streching sym/CH2 bending |
| 1111 | CH3 rocking |
| 934 | C−C(O) stretching mode (amide IV) |
| 721 | CH2 rocking/C=O deformation |
| 678 | NH out-of-plane mode (amide V) |
| Property | Sample | 0 h | 2 h | 24 h | 360 h | 744 h | 1656 h |
|---|---|---|---|---|---|---|---|
| Tg (°C) | PA11-HR | 44.49 | 42.34 | 40.62 | 39.34 | 39.78 | 39.89 |
| Tm (°C) | 193.9 | 194.98 | 197.89 | 194.3 | 193.28 | 195.16 | |
| ∆H (J/g) | 51.87 | 52.55 | 45.7 | 51 | 48.22 | 57.83 | |
| χc (%) | 23.16 | 23.46 | 20.4 | 22.77 | 21.53 | 25.82 | |
| Tg (°C) | PA11-WA | 44.49 | 42.57 | 42.83 | 42.07 | 40.59 | 41.22 |
| Tm (°C) | 193.9 | 195.02 | 194.67 | 194.62 | 195.25 | 195.16 | |
| ∆H (J/g) | 51.87 | 50.27 | 44.88 | 44.84 | 48.43 | 57 | |
| χc (%) | 23.16 | 22.44 | 20.03 | 20.02 | 21.62 | 25.45 | |
| Tg (°C) | PA12-HR | 42.77 | 41.89 | 41.61 | 39.84 | 37.71 | 39.33 |
| Tm (°C) | 178.86 | 180.94 | 185.94 | 184.14 | 182.96 | 184.88 | |
| ∆H (J/g) | 46.52 | 50.13 | 40.11 | 33.26 | 47.09 | 45.83 | |
| χc (%) | 49.48 | 52.77 | 42.22 | 35.01 | 49.57 | 48.25 | |
| Tg (°C) | PA12-WA | 42.77 | 43.35 | 47.03 | 48.01 | 40.92 | 39.95 |
| Tm (°C) | 178.86 | 184.03 | 182.66 | 182.06 | 182.46 | 184.2 | |
| ∆H (J/g) | 46.52 | 43 | 34.56 | 46.17 | 39.49 | 46.64 | |
| χc (%) | 49.48 | 45.26 | 36.38 | 48.6 | 41.57 | 49.09 |
| Time (h) | PA11-RH | PA12-RH | PA11-W | PA12-W |
|---|---|---|---|---|
| 0 | 74 | 73 | 74 | 73 |
| 24 | 74 | 71 | 74 | 73 |
| 360 | 74 | 72 | 74 | 73 |
| 744 | 74 | 72 | 74 | 71 |
| 1656 | 73 | 74 | 72 | 72 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bahrami, M.; Abenojar, J.; Martínez, M.A. Comparative Characterization of Hot-Pressed Polyamide 11 and 12: Mechanical, Thermal and Durability Properties. Polymers 2021, 13, 3553. https://doi.org/10.3390/polym13203553
Bahrami M, Abenojar J, Martínez MA. Comparative Characterization of Hot-Pressed Polyamide 11 and 12: Mechanical, Thermal and Durability Properties. Polymers. 2021; 13(20):3553. https://doi.org/10.3390/polym13203553
Chicago/Turabian StyleBahrami, Mohsen, Juana Abenojar, and Miguel Angel Martínez. 2021. "Comparative Characterization of Hot-Pressed Polyamide 11 and 12: Mechanical, Thermal and Durability Properties" Polymers 13, no. 20: 3553. https://doi.org/10.3390/polym13203553