United-Atom Molecular Dynamics Study of the Mechanical and Thermomechanical Properties of an Industrial Epoxy
Abstract
:1. Introduction
2. Experimental Methods
3. Molecular Model
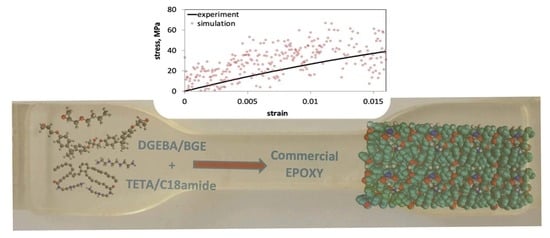
4. Results and Discussion
4.1. Experimental
4.2. Computational
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pascault, J.P.; Williams, R. Epoxy Polymers New Materials and Innovations; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Jin, F.-L.; Li, X.; Park, S.-J. Synthesis and Application of Epoxy Resins: A Review. J. Ind. Eng. Chem. 2015, 29, 1–11. [Google Scholar] [CrossRef]
- Lapique, F.; Redford, K. Curing Effects on Viscosity and Mechanical Properties of a Commercial Epoxy Resin Adhesive. Int. J. Adhes. Adhes. 2002, 22, 337–346. [Google Scholar] [CrossRef]
- Havlíček, I.; Dušek, K. Crosslinked Epoxies. In Proceedings of the 9th Discussion Conference, Prague, Czech Republic, 14–17 July 1986; pp. 417–424. [Google Scholar] [CrossRef]
- Vella, D.; Mahadevan, L. A Simple Microscopic Model for the Dynamics of Adhesive Failure. Langmuir 2006, 22, 163–168. [Google Scholar] [CrossRef] [Green Version]
- Yarovsky, I.; Evans, E. Computer Simulation of Structure and Properties of Crosslinked Polymers: Application to Epoxy Resins. Polymer 2002, 43, 963–969. [Google Scholar] [CrossRef]
- Odegard, G.M.; Jensen, B.D.; Gowtham, S.; Wu, J.; He, J. Predicting Mechanical Response of Crosslinked Epoxy Using ReaxFF. Chem. Phys. Lett. 2014, 591, 175–178. [Google Scholar] [CrossRef] [Green Version]
- Unger, R.; Braun, U.; Fankhänel, J.; Daum, B.; Arash, B.; Rolfes, R. Molecular Modelling of Epoxy Resin Crosslinking Experimentally Validated by Near-Infrared Spectroscopy. Comput. Mater. Sci. 2019, 161, 223–235. [Google Scholar] [CrossRef]
- Shokuhfar, A.; Arab, B. The Effect of Cross Linking Density on the Mechanical Properties and Structure of the Epoxy Polymers: Molecular Dynamics Simulation. J. Mol. Modeling 2013, 19, 3719–3731. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Odegard, G.M. Molecular Modeling of Crosslink Distribution in Epoxy Polymers. Model. Simul. Mater. Sci. Eng. 2012, 20, 045018. [Google Scholar] [CrossRef]
- Wu, C.; Xu, W. Atomistic Molecular Modelling of Crosslinked Epoxy Resin. Polymer 2006, 47, 6004–6009. [Google Scholar] [CrossRef]
- Tack, J.L.; Ford, D.M. Thermodynamic and Mechanical Properties of Epoxy Resin DGEBF Crosslinked with DETDA by Molecular Dynamics. J. Mol. Graph. Model. 2008, 26, 1269–1275. [Google Scholar] [CrossRef]
- Arab, B.; Shokuhfar, A. Molecular Dynamics Simulation of Cross-Linked Epoxy Polymers: The Effect of Force Field on the Estimation of Properties. J. Nano-Electron. Phys. 2013, 5, 01013. [Google Scholar]
- Fu, Y.; Michopoulos, J.G.; Song, J.H. On Investigating the Thermomechanical Properties of Cross-Linked Epoxy via Molecular Dynamics Analysis. Nanoscale Microscale Thermophys. Eng. 2017, 21, 8–25. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Valavala, P.K.; Clancy, T.C.; Wise, K.E.; Odegard, G.M. Molecular Modeling of Crosslinked Epoxy Polymers: The Effect of Crosslink Density on Thermomechanical Properties. Polymer 2011, 52, 2445–2452. [Google Scholar] [CrossRef]
- Fan, H.B.; Yuen, M.M.F. Material Properties of the Cross-Linked Epoxy Resin Compound Predicted by Molecular Dynamics Simulation. Polymer 2007, 48, 2174–2178. [Google Scholar] [CrossRef]
- Kallivokas, S.V.; Sgouros, A.P.; Theodorou, D.N. Molecular Dynamics Simulations of EPON-862/DETDA Epoxy Networks: Structure, Topology, Elastic Constants, and Local Dynamics. Soft Matter 2019, 15, 721–733. [Google Scholar] [CrossRef]
- Shenogina, N.B.; Tsige, M.; Patnaik, S.S.; Mukhopadhyay, S.M. Molecular Modeling of Elastic Properties of Thermosetting Polymers Using a Dynamic Deformation Approach. Polymer 2013, 54, 3370–3376. [Google Scholar] [CrossRef]
- Frick, B.; Richter, D. The Microscopic Basis of the Glass Transition in Polymers from Neutron Scattering Studies. Science 1995, 267, 1939–1945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Strachan, A. Molecular Dynamics Predictions of Thermal and Mechanical Properties of Thermoset Polymer EPON862/DETDA. Polymer 2011, 52, 2920–2928. [Google Scholar] [CrossRef]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An Advanced Semantic Chemical Editor, Visualization, and Analysis Platform. Available online: http://www.jcheminf.com/content/4/1/17 (accessed on 13 August 2020).
- Plimpton, S. Fast Parallel Algorithms for Short-Range Molecular Dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Jorgensen, W.L.; Tirado-Rives, J. The OPLS [Optimized Potentials for Liquid Simulations] Potential Functions for Proteins, Energy Minimizations for Crystals of Cyclic Peptides and Crambin. J. Am. Chem. Soc. 1988, 110, 1657–1666. [Google Scholar] [CrossRef]
- Tamir, E.; Srebnik, S.; Sidess, A. Prediction of the Relaxation Modulus of a Fluoroelastomer Using Molecular Dynamics Simulation. Chem. Eng. Sci. 2020, 225, 115786. [Google Scholar] [CrossRef]
- Aramoon, A.; Breitzman, T.D.; Woodward, C.; El-Awady, J.A. Correlating Free-Volume Hole Distribution to the Glass Transition Temperature of Epoxy Polymers. Ind. Eng. Chem. Res. 2017, 121, 8399–8407. [Google Scholar] [CrossRef]
- Vashisth, A.; Ashraf, C.; Bakis, C.E.; van Duin, A.C.T. Effect of Chemical Structure on Thermo-Mechanical Properties of Epoxy Polymers: Comparison of Accelerated ReaxFF Simulations and Experiments. Polymer 2018, 158, 354–363. [Google Scholar] [CrossRef]
- Littell, J.D.; Ruggeri, C.R.; Goldberg, R.K.; Roberts, G.D.; Arnold, W.A.; Binienda, W.K. Measurement of Epoxy Resin Tension, Compression, and Shear Stress–Strain Curves over a Wide Range of Strain Rates Using Small Test Specimens. J. Aerosp. Eng. 2008, 21, 162–173. [Google Scholar] [CrossRef]
- Ting, T.; Chen, T. Poisson’s Ratio for Anisotropic Elastic Materials Can Have No Bounds. Q. J. Mech. Appl. Math. 2005, 58, 73–82. [Google Scholar] [CrossRef]
- Péron, M.; Sobotka, V.; Boyard, N.; Corre, S.L. Bulk Modulus Evolution of Thermoset Resins during Crosslinking: Is a Direct and Accurate Measurement Possible? J. Compos. Mater. 2016, 51, 463–477. [Google Scholar] [CrossRef] [Green Version]
- Tamir, E.; Sidess, A.; Srebnik, S. Thermodynamic, Structural, and Mechanical Properties of Fluoropolymers from Molecular Dynamics Simulation: Comparison of Force Fields. Chem. Eng. Sci. 2019, 205, 332–340. [Google Scholar] [CrossRef]
- Gavrielides, A.; Duguet, T.; Aufray, M.; Lacaze-Dufaure, C. Model of the DGEBA-EDA Epoxy Polymer: Experiments and Simulation Using Classical Molecular Dynamics. Int. J. Polym. Sci. 2019, 2019, 9604714. [Google Scholar] [CrossRef] [Green Version]
- Eslami, H.; Kesik, M.; Karimi-Varzaneh, H.A.; Müller-Plathe, F. Sorption and Diffusion of Carbon Dioxide and Nitrogen in Poly(Methyl Methacrylate). J. Chem. Phys. 2013, 139, 124902. [Google Scholar] [CrossRef]
- Afzal, M.A.F.; Browning, A.R.; Goldberg, A.; Halls, M.D.; Gavartin, J.L.; Morisato, T.; Hughes, T.F.; Giesen, D.J.; Goose, J.E. High-Throughput Molecular Dynamics Simulations and Validation of Thermophysical Properties of Polymers for Various Applications. ACS Appl. Polym. Mater. 2021, 3, 620–630. [Google Scholar] [CrossRef]
- Gupta, V.B.; Drzal, L.T.; Lee, C.Y.C.; Rich, M.J. The Temperature-Dependence of Some Mechanical Properties of a Cured Epoxy Resin System. Polym. Eng. Sci. 1985, 25, 812–823. [Google Scholar] [CrossRef]








| −5 °C | RT | 60 °C | |
|---|---|---|---|
| Modulus [GPa] | 3.0 | 2.6 | 0.3 |
| Tensile strength [MPa] | 2.6 | 40 | 22 |
| Poisson’s ratio | 0.39 | 0.37 | 0.45 |
| Elongation at failure [%] | 0.7 | 1.7 | 1.6 |
| After 7 Days | After 1 Year | |
|---|---|---|
| Tg [°C] first heating cycle | 43 | 57 |
| Tg [°C] second heating cycle | 49 | 60 |
| Compound | Density [g/cm3] | Calculated Density [g/cm3] |
|---|---|---|
| DGEBA | 1.13 | 1.156 ± 0.003 |
| BGE | 0.91 | 0.897 ± 0.004 |
| Epikure 3140 | 0.97 | 0.965 ± 0.005 |
| EPON | 1.10 | 1.126 ± 0.008 |
| Epoxy | 1.12 | 1.117 ± 0.003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maicas, R.; Yungerman, I.; Weber, Y.B.; Srebnik, S. United-Atom Molecular Dynamics Study of the Mechanical and Thermomechanical Properties of an Industrial Epoxy. Polymers 2021, 13, 3443. https://doi.org/10.3390/polym13193443
Maicas R, Yungerman I, Weber YB, Srebnik S. United-Atom Molecular Dynamics Study of the Mechanical and Thermomechanical Properties of an Industrial Epoxy. Polymers. 2021; 13(19):3443. https://doi.org/10.3390/polym13193443
Chicago/Turabian StyleMaicas, Riki, Irena Yungerman, Yarden B. Weber, and Simcha Srebnik. 2021. "United-Atom Molecular Dynamics Study of the Mechanical and Thermomechanical Properties of an Industrial Epoxy" Polymers 13, no. 19: 3443. https://doi.org/10.3390/polym13193443