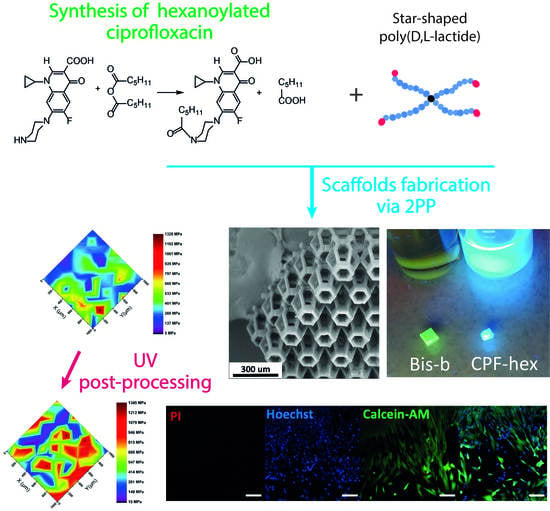
A Hydrophobic Derivative of Ciprofloxacin as a New Photoinitiator of Two-Photon Polymerization: Synthesis and Usage for the Formation of Biocompatible Polylactide-Based 3D Scaffolds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of CPF-hex
2.2. Preparation of Photocurable Resins
2.3. Scaffolds Fabrication via 2PP
2.4. Analytical Methods
2.4.1. Spectroscopic Methods
2.4.2. Optical Microscopy SEM
2.4.3. DSC
2.4.4. Nanoindentation
2.5. Cytotoxicity
3. Results and Discussion
3.1. Synthesis of CPF-hex Optical Properties 2PP Fabrication
3.2. IR Spectra and Differential Scanning Calorimetry
3.3. Mechanical Properties
3.4. Cytotoxicity of Scaffolds with CPF-hex or Bis-b
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Emons, M.; Obata, K.; Binhammer, T.; Ovsianikov, A.; Chichkov, B.N.; Morgner, U. Two-photon polymerization technique with sub-50 nm resolution by sub-10 fs laser pulses. Opt. Mater. Express 2012, 2, 942–947. [Google Scholar] [CrossRef]
- Demina, T.S.; Bardakova, K.N.; Minaev, N.V.; Svidchenko, E.A.; Istomin, A.V.; Goncharuk, G.P.; Vladimirov, L.V.; Grachev, A.V.; Zelenetskii, A.N.; Timashev, P.S.; et al. Two-photon-induced microstereolithography of chitosan-g-oligolactides as a function of their stereochemical composition. Polymers 2017, 9, 302. [Google Scholar] [CrossRef] [PubMed]
- Perevoznik, D.; Nazir, R.; Kiyan, R.; Kurselis, K.; Koszarna, B.; Gryko, D.T.; Chichkov, B.N. High-speed two-photon polymerization 3D printing with a microchip laser at its fundamental wavelength. Opt. Express 2019, 27, 25119–25125. [Google Scholar] [CrossRef] [PubMed]
- Mao, M.; He, J.; Li, X.; Zhang, B.; Lei, Q.; Liu, Y.; Li, D. The emerging frontiers and applications of high-resolution 3D printing. Micromachines 2017, 8, 113. [Google Scholar] [CrossRef]
- Ovsianikov, A. Investigation of Two-Photon Polymerization Technique for Applications in Photonics and Biomedicine; Cuvillier Verlag: Göttingen, Germany, 2009; ISBN 3736929161. [Google Scholar]
- Stampfl, J.; Liska, R.; Ovsianikov, A. Multiphoton Lithography: Techniques, Materials, and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2016; ISBN 3527337172. [Google Scholar]
- Heller, C.; Pucher, N.; Seidl, B.; Kalinyaprak-Icten, K.; Ullrich, G.; Kuna, L.; Satzinger, V.; Schmidt, V.; Lichtenegger, H.C.; Stampfl, J. One-and two-photon activity of cross-conjugated photoinitiators with bathochromic shift. J. Polym. Sci. Part A Polym. Chem. 2007, 45, 3280–3291. [Google Scholar] [CrossRef]
- Li, Z.; Pucher, N.; Cicha, K.; Torgersen, J.; Ligon, S.C.; Ajami, A.; Husinsky, W.; Rosspeintner, A.; Vauthey, E.; Naumov, S. A straightforward synthesis and structure–activity relationship of highly efficient initiators for two-photon polymerization. Macromolecules 2013, 46, 352–361. [Google Scholar] [CrossRef]
- Weisgrab, G.; Guillaume, O.; Guo, Z.; Heimel, P.; Slezak, P.; Poot, A.; Grijpma, D.; Ovsianikov, A. 3D Printing of large-scale and highly porous biodegradable tissue engineering scaffolds from poly (trimethylene-carbonate) using two-photon-polymerization. Biofabrication 2020, 12, 45036. [Google Scholar] [CrossRef] [PubMed]
- Whitby, R.; Ben-Tal, Y.; MacMillan, R.; Janssens, S.; Raymond, S.; Clarke, D.; Jin, J.; Kay, A.; Simpson, M.C. Photoinitiators for two-photon polymerisation: Effect of branching and viscosity on polymerisation thresholds. RSC Adv. 2017, 7, 13232–13239. [Google Scholar] [CrossRef] [Green Version]
- Xing, J.-F.; Chen, W.-Q.; Dong, X.-Z.; Tanaka, T.; Fang, X.-Y.; Duan, X.-M.; Kawata, S. Synthesis, optical and initiating properties of new two-photon polymerization initiators: 2, 7-Bis (styryl) anthraquinone derivatives. J. Photochem. Photobiol. A Chem. 2007, 189, 398–404. [Google Scholar] [CrossRef]
- Arnoux, C.; Konishi, T.; Van Elslande, E.; Poutougnigni, E.-A.; Mulatier, J.-C.; Khrouz, L.; Bucher, C.; Dumont, E.; Kamada, K.; Andraud, C. Polymerization photoinitiators with near-resonance enhanced two-photon absorption cross-Section: Toward high-resolution photoresist with improved sensitivity. Macromolecules 2020, 53, 9264–9278. [Google Scholar] [CrossRef]
- You, S.; Li, J.; Zhu, W.; Yu, C.; Mei, D.; Chen, S. Nanoscale 3D printing of hydrogels for cellular tissue engineering. J. Mater. Chem. B 2018, 6, 2187–2197. [Google Scholar] [CrossRef]
- Tromayer, M.; Gruber, P.; Rosspeintner, A.; Ajami, A.; Husinsky, W.; Plasser, F.; González, L.; Vauthey, E.; Ovsianikov, A.; Liska, R. Wavelength-optimized two-photon polymerization using initiators based on multipolar aminostyryl-1, 3, 5-triazines. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Nguyen, A.K.; Narayan, R.J. Two-photon polymerization for biological applications. Mater. Today 2017, 20, 314–322. [Google Scholar] [CrossRef]
- Malval, J.-P.; Jin, M.; Morlet-Savary, F.; Chaumeil, H.; Defoin, A.; Soppera, O.; Scheul, T.; Bouriau, M.; Baldeck, P.L. Enhancement of the two-photon initiating efficiency of a thioxanthone derivative through a chevron-shaped architecture. Chem. Mater. 2011, 23, 3411–3420. [Google Scholar] [CrossRef]
- Nazir, R.; Danilevicius, P.; Ciuciu, A.I.; Chatzinikolaidou, M.; Gray, D.; Flamigni, L.; Farsari, M.; Gryko, D.T. π-expanded ketocoumarins as efficient, biocompatible initiators for two-photon-induced polymerization. Chem. Mater. 2014, 26, 3175–3184. [Google Scholar] [CrossRef]
- Snowberger, S.; Adejumo, H.; He, K.; Mangalgiri, K.P.; Hopanna, M.; Soares, A.D.; Blaney, L. Direct photolysis of fluoroquinolone antibiotics at 253.7 nm: Specific reaction kinetics and formation of equally potent fluoroquinolone antibiotics. Environ. Sci. Technol. 2016, 50, 9533–9542. [Google Scholar] [CrossRef]
- Soldevila, S.; Cuquerella, M.C.; Lhiaubet-Vallet, V.; Edge, R.; Bosca, F. Seeking the mechanism responsible for fluoroquinolone photomutagenicity: A pulse radiolysis, steady-state, and laser flash photolysis study. Free Radic. Biol. Med. 2014, 67, 417–425. [Google Scholar] [CrossRef]
- Sturini, M.; Speltini, A.; Maraschi, F.; Pretali, L.; Profumo, A.; Fasani, E.; Albini, A.; Migliavacca, R.; Nucleo, E. Photodegradation of fluoroquinolones in surface water and antimicrobial activity of the photoproducts. Water Res. 2012, 46, 5575–5582. [Google Scholar] [CrossRef]
- Timashev, P.; Kuznetsova, D.; Koroleva, A.; Prodanets, N.; Deiwick, A.; Piskun, Y.; Bardakova, K.; Dzhoyashvili, N.; Kostjuk, S.; Zagaynova, E.; et al. Novel biodegradable star-shaped polylactide scaffolds for bone regeneration fabricated by two-photon polymerization. Nanomedicine 2016, 11, 1041–1053. [Google Scholar] [CrossRef]
- Tomé, L.C.; Freire, M.G.; Rebelo, L.P.N.; Silvestre, A.J.D.; Neto, C.P.; Marrucho, I.M.; Freire, C.S.R. Surface hydrophobization of bacterial and vegetable cellulose fibers using ionic liquids as solvent media and catalysts. Green Chem. 2011, 13, 2464–2470. [Google Scholar] [CrossRef] [Green Version]
- Bardakova, K.N.; Grebenik, E.A.; Minaev, N.V.; Churbanov, S.N.; Moldagazyeva, Z. Tailoring the collagen film structural properties via direct laser crosslinking of star-shaped polylactide for robust scaffold formation. Mater. Sci. Eng. C 2020, 107, 110300. [Google Scholar] [CrossRef]
- Shin, C.-S.; Li, T.-J.; Lin, C.-L. Alleviating distortion and improving the Young’s modulus in two-photon polymerization fabrications. Micromachines 2018, 9, 615. [Google Scholar] [CrossRef] [Green Version]
- Purtov, J.; Verch, A.; Rogin, P.; Hensel, R. Improved development procedure to enhance the stability of microstructures created by two-photon polymerization. Microelectron. Eng. 2018, 194, 45–50. [Google Scholar] [CrossRef]
- Steyrer, B.; Neubauer, P.; Liska, R.; Stampfl, J. Visible light photoinitiator for 3D-printing of tough methacrylate resins. Materials 2017, 10, 1445. [Google Scholar] [CrossRef] [Green Version]
- LaFratta, C.N.; Baldacchini, T. Two-photon polymerization metrology: Characterization methods of mechanisms and microstructures. Micromachines 2017, 8, 101. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Zhang, Y.; Gautam, S.; Liu, L.; Dey, J.; Chen, W.; Mason, R.P.; Serrano, C.A.; Schug, K.A.; Tang, L. Development of aliphatic biodegradable photoluminescent polymers. Proc. Natl. Acad. Sci. USA 2009, 106, 10086–10091. [Google Scholar] [CrossRef] [Green Version]
- Shavkuta, B.; Bardakova, K.; Khristidis, Y.; Minaev, N.V.; Frolova, A.; Kotova, S.; Aksenova, N.; Heydari, Z.; Semenova, E.; Khlebnikova, T. Approach to tune drug release in particles fabricated from methacrylate functionalized polylactides. Mol. Syst. Des. Eng. 2021, 6, 202–213. [Google Scholar] [CrossRef]
- Shpichka, A.; Koroleva, A.; Kuznetsova, D.; Dmitriev, R.I.; Timashev, P. Fabrication and handling of 3D scaffolds based on polymers and decellularized tissues. Multi-Parametr. Live Cell Microsc. 3D Tissue Model. 2017, 1035, 71–81. [Google Scholar]
- Froimowitz, M. HyperChem: A software package for computational chemistry and molecular modeling. Biotechniques 1993, 14, 1010–1013. [Google Scholar] [PubMed]
- Albini, A.; Monti, S. Photophysics and photochemistry of fluoroquinolones. Chem. Soc. Rev. 2003, 32, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Zandrini, T.; Liaros, N.; Jiang, L.J.; Lu, Y.F.; Fourkas, J.T.; Osellame, R.; Baldacchini, T. Effect of the resin viscosity on the writing properties of two-photon polymerization. Opt. Mater. Express 2019, 9, 2601–2616. [Google Scholar] [CrossRef]
- Skliutas, E.; Lebedevaite, M.; Kabouraki, E.; Baldacchini, T.; Ostrauskaite, J.; Vamvakaki, M.; Farsari, M.; Juodkazis, S.; Malinauskas, M. Polymerization mechanisms initiated by spatio-temporally confined light. Nanophotonics 2021, 10, 1211–1242. [Google Scholar] [CrossRef]
- Rieutord, A.; Vazquez, L.; Soursac, M.; Prognon, P.; Blais, J.; Bourget, P.; Mahuzier, G. Fluoroquinolones as sensitizers of lanthanide fluorescence: Application to the liquid chromatographic determination of ciprofloxacin using terbium. Anal. Chim. Acta 1994, 290, 215–225. [Google Scholar] [CrossRef]
- Xie, S.; Manuguri, S.; Ramström, O.; Yan, M. Impact of hydrogen bonding on the fluorescence of N-Amidinated fluoroquinolones. Chem. Asian J. 2019, 14, 910–916. [Google Scholar] [CrossRef]
- Mazumdar, P.; Das, D.; Sahoo, G.P.; Salgado-Morán, G.; Misra, A. Aggregation induced emission enhancement of 4, 4′-bis (diethylamino) benzophenone with an exceptionally large blue shift and its potential use as glucose sensor. Phys. Chem. Chem. Phys. 2015, 17, 3343–3354. [Google Scholar] [CrossRef]
- Flamourakis, G.; Kordas, A.; Barmparis, G.D.; Ranella, A.; Farsari, M. Low-autofluorescence, transparent composite for multiphoton 3D printing. Opt. Mater. Express 2021, 11, 801. [Google Scholar] [CrossRef]
- Lambrecht, G.; Mallol, C. Autofluorescence of experimentally heated bone: Potential archaeological applications and relevance for estimating degree of burning. J. Archaeol. Sci. Rep. 2020, 31, 102333. [Google Scholar] [CrossRef]
- Teixeira, S.; Eblagon, K.M.; Miranda, F.; Pereira, M.F.R.; Figueiredo, J.L. Towards controlled degradation of poly(lactic) acid in technical applications. C 2021, 7, 42. [Google Scholar] [CrossRef]
- Zou, H.; Yi, C.; Wang, L.; Liu, H.; Xu, W. Thermal degradation of poly (lactic acid) measured by thermogravimetry coupled to Fourier transform infrared spectroscopy. J. Therm. Anal. Calorim. 2009, 97, 929–935. [Google Scholar] [CrossRef]
- Sabree, I.; Gough, J.E.; Derby, B. Mechanical properties of porous ceramic scaffolds: Influence of internal dimensions. Ceram. Int. 2015, 41, 8425–8432. [Google Scholar] [CrossRef]
- Wang, L.; Wang, C.; Wu, S.; Fan, Y.; Li, X. Influence of the mechanical properties of biomaterials on degradability, cell behaviors and signaling pathways: Current progress and challenges. Biomater. Sci. 2020, 8, 2714–2733. [Google Scholar] [CrossRef]
- Yousif, E.; Haddad, R. Photodegradation and photostabilization of polymers, especially polystyrene: Review. Springerplus 2013, 2, 398. [Google Scholar] [CrossRef] [Green Version]
- Gibson, L.J. The mechanical behaviour of cancellous bone. J. Biomech. 1985, 18, 317–328. [Google Scholar] [CrossRef]










| Sample | Tg, °C | T10% massloss, °C | Moisture Content at 100 °C, % |
|---|---|---|---|
| PLA | 59.7 | 314 | 8 |
| [PLA + CPF-hex] after 2PP | 56.1 | 315 | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bardakova, K.N.; Faletrov, Y.V.; Epifanov, E.O.; Minaev, N.V.; Kaplin, V.S.; Piskun, Y.A.; Koteneva, P.I.; Shkumatov, V.M.; Aksenova, N.A.; Shpichka, A.I.; et al. A Hydrophobic Derivative of Ciprofloxacin as a New Photoinitiator of Two-Photon Polymerization: Synthesis and Usage for the Formation of Biocompatible Polylactide-Based 3D Scaffolds. Polymers 2021, 13, 3385. https://doi.org/10.3390/polym13193385
Bardakova KN, Faletrov YV, Epifanov EO, Minaev NV, Kaplin VS, Piskun YA, Koteneva PI, Shkumatov VM, Aksenova NA, Shpichka AI, et al. A Hydrophobic Derivative of Ciprofloxacin as a New Photoinitiator of Two-Photon Polymerization: Synthesis and Usage for the Formation of Biocompatible Polylactide-Based 3D Scaffolds. Polymers. 2021; 13(19):3385. https://doi.org/10.3390/polym13193385
Chicago/Turabian StyleBardakova, Kseniia N., Yaroslav V. Faletrov, Evgenii O. Epifanov, Nikita V. Minaev, Vladislav S. Kaplin, Yuliya A. Piskun, Polina I. Koteneva, Vladimir M. Shkumatov, Nadezhda A. Aksenova, Anastasia I. Shpichka, and et al. 2021. "A Hydrophobic Derivative of Ciprofloxacin as a New Photoinitiator of Two-Photon Polymerization: Synthesis and Usage for the Formation of Biocompatible Polylactide-Based 3D Scaffolds" Polymers 13, no. 19: 3385. https://doi.org/10.3390/polym13193385