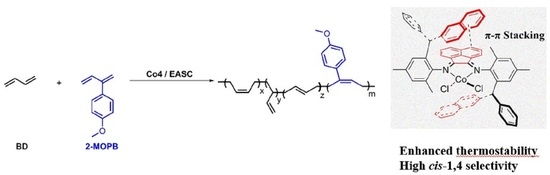
cis-1,4 Selective Coordination Polymerization of 1,3-Butadiene and Copolymerization with Polar 2-(4-Methoxyphenyl)-1,3-butadiene by Acenaphthene-Based α-Diimine Cobalt Complexes Featuring Intra-Ligand π-π Stacking Interactions
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Considerations and Materials
2.2. Typical Procedure for Butadiene Homopolymerization and Copolymerization
2.3. Synthesis of Complexes (Co1–Co4)
2.3.1. Synthesis of Complex Co1
2.3.2. Synthesis of Complex Co2
2.3.3. Synthesis of Complex Co3
2.3.4. Synthesis of Complex Co4
3. Results
3.1. Synthesis and Characterization of the Cobalt Complexes
3.2. 1,3-Butadiene Homopolymerization
3.3. Copolymerization of 2-(4-Methoxyphenyl)-1,3-butadiene with 1,3-Butadiene
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wilson, D. Recent advances in the neodymium catalysed polymerisation of 1,3-dienes. Makromol. Chem. Symp. 1993, 66, 273. [Google Scholar] [CrossRef]
- Friebe, L.; Nuyken, O.; Obrecht, W. Neodymium-Based Ziegler/Natta Catalysts and their Application in Diene Polymerization. In Neodymium Based Ziegler Catalysts—Fundamental Chemistry; Nuyken, O., Ed.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 1–154. [Google Scholar]
- Ricci, G.; Sommazzi, A.; Masi, F.; Ricci, M.; Boglia, A.; Leone, G. Well-defined transition metal complexes with phosphorus and nitrogen ligands for 1,3-dienes polymerization. Coord. Chem. Rev. 2010, 254, 661–676. [Google Scholar] [CrossRef]
- Boffa, L.S.; Novak, B. Copolymerization of Polar Monomers with Olefins Using Transition-Metal Complexes. Chem. Rev. 2000, 100, 1479–1494. [Google Scholar] [CrossRef] [PubMed]
- Amin, S.B.; Marks, T.J. Versatile Pathways for In Situ Polyolefin Functionalization with Heteroatoms: Catalytic Chain Transfer. Angew. Chem. Int. Ed. 2008, 47, 2006–2025. [Google Scholar] [CrossRef]
- Chen, E.Y.-X. Coordination Polymerization of Polar Vinyl Monomers by Single-Site Metal Catalysts. Chem. Rev. 2009, 109, 5157–5214. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.K.; Killian, C.M.; Brookhart, M. New Pd(II)- and Ni(II)-Based Catalysts for Polymerization of Ethylene and .alpha.-Olefins. J. Am. Chem. Soc. 1995, 117, 6414–6415. [Google Scholar] [CrossRef]
- Johnson, L.K.; Mecking, S.; Brookhart, M. Copolymerization of Ethylene and Propylene with Functionalized Vinyl Monomers by Palladium(II) Catalysts. J. Am. Chem. Soc. 1996, 118, 267–268. [Google Scholar] [CrossRef]
- Wang, T.; Wu, C.; Cui, D. cis-1,4 Selective Copolymerization of Butadiene and Functionalized α-Olefins via Polar Group Activation Mechanism. Macromolecules 2020, 53, 6380–6386. [Google Scholar] [CrossRef]
- Leicht, H.; Göttker-Schnetmann, I.; Mecking, S. Stereoselective Copolymerization of Butadiene and Functionalized 1,3-Dienes with Neodymium-Based Catalysts. Macromolecules 2017, 50, 8464–8468. [Google Scholar] [CrossRef]
- Yao, C.; Liu, N.; Long, S.; Wu, C.; Cui, D. Highly cis-1,4-selective coordination polymerization of polar 2-(4-methoxyphenyl)-1,3-butadiene and copolymerization with isoprene using a β-diketiminato yttrium bis(alkyl) complex. Polym. Chem. 2015, 7, 1264–1270. [Google Scholar] [CrossRef]
- Shi, Z.; Guo, F.; Meng, R.; Jiang, L.; Li, Y. Stereoselective copolymerization of amino-functionalized styrene with butadiene using a half-sandwich scandium complex. Polym. Chem. 2016, 7, 7365–7369. [Google Scholar] [CrossRef]
- Buonerba, A.; Cuomo, C.; Speranza, V.; Grassi, A. Crystalline Syndiotactic Polystyrene as Reinforcing Agent of cis-1,4-Polybutadiene Rubber. Macromolecules 2010, 43, 367–374. [Google Scholar] [CrossRef]
- Gong, D.; Tang, F.; Xu, Y.; Hu, Z.; Luo, W. Cobalt catalysed controlled copolymerization: An efficient approach to bifunctional polyisoprene with enhanced properties. Polym. Chem. 2021, 12, 1653–1660. [Google Scholar] [CrossRef]
- Xu, Y.; Zhao, J.; Gan, Q.; Ying, W.; Hu, Z.; Tang, F.; Luo, W.; Luo, Y.; Jian, Z.; Gong, D. Synthesis and properties investigation of hydroxyl functionalized polyisoprene prepared by cobalt catalyzed co-polymerization of isoprene and hydroxylmyrcene. Polym. Chem. 2020, 11, 2034–2043. [Google Scholar] [CrossRef]
- Tempel, D.J.; Johnson, L.K.; Huff, R.L.; White, P.S.; Brookhart, M. Mechanistic Studies of Pd(II)−α-Diimine-Catalyzed Olefin Polymerizations1. J. Am. Chem. Soc. 2000, 122, 6686–6700. [Google Scholar] [CrossRef]
- Wang, X.; Dong, B.; Yang, Q.; Liu, H.; Hu, Y.; Zhang, X. Boosting the Thermal Stability of α-Diimine Palladium Complexes in Norbornene Polymerization from Construction of Intraligand Hydrogen Bonding and Simultaneous Increasing Axial/Equatorial Bulkiness. Inorg. Chem. 2021, 60, 2347–2361. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Li, S.; Tan, C.; Kong, W.; Xu, G.; Zhang, S.; Liu, B.; Dai, S. π–π interaction effect in insertion polymerization with α-Diimine palladium systems. J. Catal. 2019, 378, 184–191. [Google Scholar] [CrossRef]
- Xiao, Z.; Zheng, H.; Du, C.; Zhong, L.; Liao, H.; Gao, J.; Gao, H.; Wu, Q. Enhancement on Alternating Copolymerization of Carbon Monoxide and Styrene by Dibenzobarrelene-Based α-Diimine Palladium Catalysts. Macromolecules 2018, 51, 9110–9121. [Google Scholar] [CrossRef]
- Du, C.; Zhong, L.; Gao, J.; Zhong, S.; Liao, H.; Gao, H.; Wu, Q. Living (co)polymerization of ethylene and bio-based furfuryl acrylate using dibenzobarrelene derived α-diimine palladium catalysts. Polym. Chem. 2019, 10, 2029–2038. [Google Scholar] [CrossRef]
- Wang, F.; Tanaka, R.; Li, Q.; Nakayama, Y.; Shiono, T. Chain-Walking Polymerization of Linear Internal Octenes Catalyzed by α-Diimine Nickel Complexes. Organometallics 2018, 37, 1358–1367. [Google Scholar] [CrossRef]
- Rosa, V.; Gonzalez, P.J.; Avilés, T.; Gomes, P.T.; Welter, R.; Rizzi, A.C.; Passeggi, M.C.G.; Brondino, C.D. Synthesis, Solid-State Structures, and EPR Spectroscopic Studies on Polycrystalline and Single-Crystal Samples of α-Diimine Cobalt(II) Complexes. Eur. J. Inorg. Chem. 2006, 2006, 4761–4769. [Google Scholar] [CrossRef]
- Chen, L.; Li, Y.; Yue, S.; Ling, J.; Ni, X.; Shen, Z. Chemoselective RAFT Polymerization of a Trivinyl Monomer Derived from Carbon Dioxide and 1,3-Butadiene: From Linear to Hyperbranched. Macromolecules 2017, 50, 9598–9606. [Google Scholar] [CrossRef]
- Rosa, V.; Carabineiro, S.A.; Avilés, T.; Gomes, P.T.; Welter, R.; Campos, J.M.; Ribeiro, M.D.R. Synthesis, characterisation and solid state structures of α-diimine cobalt(II) complexes: Ethylene polymerisation tests. J. Organomet. Chem. 2008, 693, 769–775. [Google Scholar] [CrossRef]
- Chakraborty, S.; Chattopadhyay, G.; Saha, C. ChemInform Abstract: A Tandem Reduction—Oxidation Protocol for the Conversion of 1-Keto-1,2,3,4-tetrahydrocarbazoles to Carbazoles via Tosylhydrazones Through Microwave Assistance: Efficient Synthesis of Glycozoline, Clausenalene, Glycozolicine, and Deoxycarbazomycin B and the Total Synthesis of Murrayafoline A. J. Heterocycl. Chem. 2013, 44, 91–98. [Google Scholar]
- Li, S.; Zhao, Y.; Dai, S. Synthesis of polyethylene thermoplastic elastomer by using robust α--diimine Ni( II ) catalysts with abundant t Bu substituents. J. Appl. Polym. Sci. 2021, 59, 638–645. [Google Scholar] [CrossRef]
- Peng, D.; Chen, C. Photoresponsive Palladium and Nickel Catalysts for Ethylene Polymerization and Copolymerization. Angew. Chem. Int. Ed. 2021. [Google Scholar] [CrossRef]
- Rosa, V.; Avilés, T.; Aullón, G.; Covelo, B.; Lodeiro, C. A New Bis(1-naphthylimino)acenaphthene Compound and Its Pd(II) and Zn(II) Complexes: Synthesis, Characterization, Solid-State Structures and Density Functional Theory Studies on the syn and anti Isomers. Inorg. Chem. 2008, 47, 7734–7744. [Google Scholar] [CrossRef]
- Fang, L.; Zhao, W.-P.; Han, C.; Zhang, C.-Y.; Liu, H.; Hu, Y.-M.; Zhang, X.-Q. 1,3-Butadiene Polymerizations Catalyzed by Cobalt and Iron Dichloride Complexes Bearing Pyrazolylimine Ligands. Chin. J. Polym. Sci. 2018, 37, 462–470. [Google Scholar] [CrossRef]
- Fang, L.; Zhao, W.; Han, C.; Liu, H.; Hu, Y.; Zhang, X. Isoprene Polymerization with Pyrazolylimine Cobalt(II) Complexes: Manipulation of 3,4-Selectivities by Ligand Design and Use of Triphenylphosphine. Eur. J. Inorg. Chem. 2019, 2019, 609–616. [Google Scholar] [CrossRef]
- Liu, H.; Zhuang, R.; Dong, B.; Wang, F.; Hu, Y.-M.; Zhang, X.-Q. Mono- and Binuclear Cobalt(II) Complexes Supported by Quinoline-2-imidate Ligands: Synthesis, Characterization, and 1,3-Butadiene Polymerization. Chin. J. Polym. Sci. 2018, 36, 943–952. [Google Scholar] [CrossRef]
- Liu, H.; Yang, S.-Z.; Wang, F.; Bai, C.-X.; Hu, Y.-M.; Zhang, X.-Q. Polymerization of 1,3-butadiene catalyzed by cobalt(II) and nickel(II) complexes bearing pyridine-2-imidate ligands. Chin. J. Polym. Sci. 2016, 34, 1060–1069. [Google Scholar] [CrossRef]
- Wang, X.; Dong, B.; Yang, Q.; Liu, H.; Zhang, C.; Zhang, X. α-Diimine nickel complexes bearing axially bulky terphenyl and equatorially bulky dibenzobarrelene groups: Synthesis, characterization and olefin polymerization studies. Polym. Chem. 2020, 11, 6783–6793. [Google Scholar] [CrossRef]







| Entry a | Cat. | EASC/[Co] | Trxn (°C) | Yield (g) | Activity b | Microstructure c | Mn d | Mw/Mn d | ||
|---|---|---|---|---|---|---|---|---|---|---|
| trans-1,4 % | cis-1,4 % | 1,2 % | ||||||||
| 1 | Co1 | 50 | 30 | 0.33 | 3.3 | 2.4 | 95.3 | 2.3 | 57.2 | 2.17 |
| 2 | Co1 | 100 | 30 | 0.42 | 4.2 | 1.6 | 95.8 | 2.6 | 35.7 | 2.04 |
| 3 | Co1 | 300 | 30 | 0.62 | 6.2 | 1.4 | 94.1 | 4.5 | 13.4 | 2.06 |
| 4 | Co1 | 600 | 30 | 0.81 | 8.1 | 1.2 | 95.8 | 3.0 | 6.8 | 2.21 |
| 5 | Co1 | 300 | 0 | 0.06 | 0.6 | 2.0 | 97.0 | 1.0 | 77.2 | 1.86 |
| 6 | Co1 | 300 | 50 | 0.64 | 6.4 | 4.6 | 93.6 | 1.8 | 19.2 | 8.56 |
| 7 | Co1 | 300 | 70 | ---- f | ---- | ---- | ---- | ---- | ---- | ---- |
| 8 | Co2 | 300 | 0 | 0.04 | 0.4 | 2.8 | 95.7 | 1.5 | 92.2 | 1.64 |
| 9 | Co2 | 300 | 30 | 0.83 | 8.3 | 1.5 | 94.9 | 3.6 | 35.0 | 2.18 |
| 10 | Co2 | 300 | 50 | 0.45 | 4.5 | 4.6 | 95.4 | 1.7 | 2.9 | 4.43 |
| 11 | Co2 | 300 | 70 | ---- f | ---- | ---- | ---- | ---- | ---- | ---- |
| 12 | Co3 | 300 | 0 | 0.02 | 0.2 | 2.0 | 96.8 | 1.2 | 91.8 | 1.77 |
| 13 | Co3 | 300 | 30 | 0.38 | 3.8 | 1.3 | 96.2 | 2.5 | 41.0 | 1.84 |
| 14 | Co3 | 300 | 50 | 0.33 | 3.3 | 2.9 | 95.3 | 1.5 | 3.2 | 2.45 |
| 15 | Co3 | 300 | 70 | ---- f | ---- | ---- | ---- | ---- | ---- | ---- |
| 16 | Co4 | 300 | 0 | 0.17 | 1.7 | 1.0 | 98.1 | 0.9 | 36.2 | 2.07 |
| 17 | Co4 | 300 | 30 | 0.83 | 8.3 | 4.3 | 91.4 | 4.3 | 7.6 | 1.88 |
| 18 | Co4 | 300 | 50 | 0.83 | 8.3 | 5.9 | 90.0 | 4.1 | 7.8 | 6.22 |
| 19 | Co4 | 300 | 70 | 0.78 | 7.8 | 9.3 | 87.4 | 3.3 | 5.7 | 8.94 |
| 20 | Co4 | 300 | 90 | 0.70 | 7.0 | 9.5 | 86.9 | 3.6 | 2.3 | 3.86 |
| 21 e | Co4 | 300 | 50 | 0.83 | 99.6 | 4.3 | 85.3 | 13.0 | 3.7 | 1.54 |
| Entry a | Cat. | [2-MOPB]/[Co] | Yield (g) | Activity b | Inc. (mol %) | Microstructure c | Mnd | Mw/Mnd | ||
|---|---|---|---|---|---|---|---|---|---|---|
| trans-1,4 % | cis-1,4 % | 1,2 % | ||||||||
| 1 | Co4 | 5:1 | 0.58 | 5.8 | 0.46 | 10.1 | 85.5 | 4.4 | 28.4 | 2.23 |
| 2 | Co4 | 10:1 | 0.46 | 4.6 | 0.53 | 11.4 | 85.4 | 3.2 | 17.4 | 2.07 |
| 3 | Co4 | 20:1 | 0.44 | 4.4 | 0.66 | 6.9 | 90.2 | 2.8 | 12.9 | 2.02 |
| 4 | Co4 | 50:1 | 0.40 | 4.0 | 1.83 | 9.4 | 88.4 | 2.2 | 5.4 | 1.97 |
 | ||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Liu, H.; Tang, T.; Zhang, X. cis-1,4 Selective Coordination Polymerization of 1,3-Butadiene and Copolymerization with Polar 2-(4-Methoxyphenyl)-1,3-butadiene by Acenaphthene-Based α-Diimine Cobalt Complexes Featuring Intra-Ligand π-π Stacking Interactions. Polymers 2021, 13, 3329. https://doi.org/10.3390/polym13193329
Wang B, Liu H, Tang T, Zhang X. cis-1,4 Selective Coordination Polymerization of 1,3-Butadiene and Copolymerization with Polar 2-(4-Methoxyphenyl)-1,3-butadiene by Acenaphthene-Based α-Diimine Cobalt Complexes Featuring Intra-Ligand π-π Stacking Interactions. Polymers. 2021; 13(19):3329. https://doi.org/10.3390/polym13193329
Chicago/Turabian StyleWang, Beibei, Heng Liu, Tao Tang, and Xuequan Zhang. 2021. "cis-1,4 Selective Coordination Polymerization of 1,3-Butadiene and Copolymerization with Polar 2-(4-Methoxyphenyl)-1,3-butadiene by Acenaphthene-Based α-Diimine Cobalt Complexes Featuring Intra-Ligand π-π Stacking Interactions" Polymers 13, no. 19: 3329. https://doi.org/10.3390/polym13193329