Molecular Assembly in Block Copolymer-Surfactant Nanoparticle Dispersions: Information on Molecular Exchange and Apparent Solubility from High-Resolution and PFG NMR
Abstract
:1. Introduction
2. Materials and Methods
3. Results
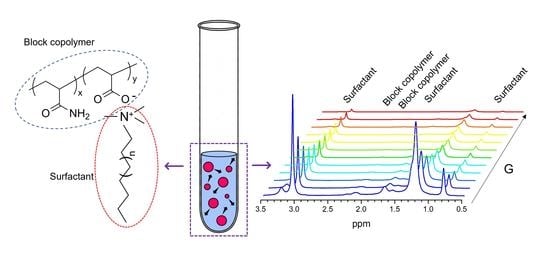
3.1. High-Resolution 1H-NMR Spectra
3.2. Compositions of Phase-Separated Samples
3.3. Self-Diffusion Measurements
4. Discussion
4.1. High-Resolution 1H NMR Spectra
4.2. Self-Diffusion Measurements
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harada, H.; Kataoka, K. Formation of Polyion Complex Micelles in an Aqueous Milieu from a Pair of Oppositely Charged Block Copolymers with Poly(ethylene glycol) Segments. Macromolecules 1995, 28, 5294–5299. [Google Scholar] [CrossRef]
- van der Burgh, S.; de Keizer, A.; Cohen Stuart, M.A. Complex Coacervation Core Micelles. Colloidal Stability and Aggregation Mechanism. Langmuir 2004, 20, 1073–1084. [Google Scholar] [CrossRef]
- Cohen Stuart, M.A.; Hofs, B.; Voets, I.K.; de Keizer, A. Assembly of polyelectrolyte-containing block copolymers in aqueous media. Curr. Opin. Colloid Interface Sci. 2005, 10, 30–36. [Google Scholar] [CrossRef]
- Voets, I.K.; de Keizer, A.; de Waard, P.; Frederik, P.M.; Bomans, P.H.H.; Schmalz, H.; Walther, A.; King, S.M.; Leermarkes, F.A.M.; Cohen Stuart, M.A. Double-Faced Micelles from Water-Soluble Polymers. Angew. Chem. Int. Ed. 2006, 45, 6673–6676. [Google Scholar] [CrossRef]
- Voets, I.K.; de Keizer, A.; Cohen Stuart, M.A. Complex coacervate core micelles. Adv. Colloid Interface Sci. 2009, 147-148, 300–318. [Google Scholar] [CrossRef]
- Ferreira, G.A.; Loh, W. Liquid crystalline nanoparticles formed by surfactant-polyelectrolyte complexes. Curr. Opin. Colloid. Int. Sci. 2017, 32, 11–22. [Google Scholar] [CrossRef]
- Berret, J.F.; Cristobal, G.; Hervé, P.; Grillo, I. Structure of colloidal complexes obtained from neutral/poly-electrolyte copolymers and oppositely charged surfactants. Eur. Phys. J. E 2002, 9, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Berret, J.F.; Hervé, P.; Aguerre-Chariol, O.; Oberdisse, J. Colloidal complexes obtained from charged block copolymers and surfactants: A comparison between small-angle neutron scattering, Cryo-TEM, and simulations. J. Phys. Chem. B 2003, 107, 8111–8118. [Google Scholar] [CrossRef]
- Hervé, P.; Destarac, M.; Berret, J.-F.; Lal, J.; Oberdisse, J.; Grillo, I. Novel Core-shell Structure for Colloids Made of Neutral/Polyelectrolyte Diblock Copolymers and Oppositely Charged Surfactants. Europhys. Lett. 2002, 58, 912–918. [Google Scholar] [CrossRef] [Green Version]
- Berret, J.-F.; Vigolo, B.; Eng, R.; Hervé, P.; Grillo, I.; Yang, L. Electrostatic Self-assembly of Oppositely Charged Copolymers and Surfactants: A Light, Neutron, and X-ray Scattering Study. Macromolecules 2004, 37, 4922–4930. [Google Scholar] [CrossRef]
- Svensson, A.; Piculell, L.; Cabane, B.; Iketi, P. A New Approach to the Phase Behavior of Oppositely Charged Polymers and Surfactants. J. Phys. Chem. B 2002, 106, 1013–1018. [Google Scholar] [CrossRef]
- Vitorazi, L.; Berret, J.-F.; Loh, W. Self-Assembly of Complex Salts of Cationic Surfactants and Anionic–Neutral Block Copolymers. Dispersions with Liquid-Crystalline Internal Structure. Langmuir 2013, 29, 14024–14033. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, G.A.; Loh, W. Addition of n-Alcohols Induces a Variety of Liquid-Crystalline Structures in Surfactant-Rich Cores of Dispersed Block Copolymer/Surfactant Nanoparticles. ACS Omega 2016, 1, 1104–1113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carneiro, N.M.; Percebom, A.M.; Loh, W. Quest for Thermoresponsive Block Copolymer Nanoparticles with Liquid–Crystalline Surfactant Cores. ACS Omega 2017, 2, 5518–5528. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, G.A.; Loh, W. Planet–Satellite Nanostructures Based on Block Copolymer-Surfactant Nanoparticles Surface-Decorated with Gold and Silver: A New Strategy for Interfacial Catalysis. Adv. Mat. Interfaces 2019, 13, 1900348. [Google Scholar] [CrossRef]
- Ferreira, G.A.; Piculell, L.; Loh, W. Hydration-dependent hierarchical structures in block copolymer-surfactant complex salts. Macromolecules 2018, 51, 9915–9924. [Google Scholar] [CrossRef]
- Price, W.S. NMR Studies of Translational Motion; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar]
- Söderman, O.; Stilbs, S. NMR Studies of Complex Surfactant Systems. Prog. Nucl. Magn. Reson. Spectrosc. 1994, 26, 445–482. [Google Scholar] [CrossRef]
- Svensson, A.; Topgaard, D.; Piculell, L.; Söderman, O. Molecular Self-Diffusion in Micellar and Discrete Cubic Phases of an Ionic Surfactant with Mixed Monovalent/Polymeric Counterions. J. Phys. Chem. B 2003, 107, 13241–13250. [Google Scholar] [CrossRef]
- Cabaleiro-Lago, C.; Nilsson, M.; Söderman, O. Self-Diffusion NMR Studies of the Host−Guest Interaction between β-Cyclodextrin and Alkyltrimethylammonium Bromide Surfactants. Langmuir 2005, 21, 11637–11644. [Google Scholar] [CrossRef]
- Bernardes, J.S.; da Silva, M.A.; Piculell, L.; Loh, W. Reverse micelles with spines: L2 phases of surfactant ion-polyion complex salts, n-alcohols and water investigated by rheology, NMR diffusion and SAXS measurements. Soft Matter 2010, 6, 144–153. [Google Scholar] [CrossRef] [Green Version]
- Percebom, A.M.; Janiak, J.; Schillén, K.; Piculell, L.; Loh, W. Micellization of water-soluble complex salts of an ionic surfactant with hairy polymeric counterions. Soft Matter 2013, 9, 515–526. [Google Scholar] [CrossRef]
- Janiak, J.; Piculell, L.; Schillén, K.; Lundberg, D. Responsive release of polyanions from soluble aggregates formed with a hydrolyzable cationic surfactant and a nonionic surfactant. Soft Matter 2013, 9, 4103–4112. [Google Scholar] [CrossRef]
- Jansson, M.; Stilbs, P. A Comparative Study of Organic Counterion Binding to Micelles with the Fourier Transform NMR Self-Diffusion Technique. J. Phys. Chem. 1985, 89, 4868–4873. [Google Scholar] [CrossRef]
- Ginley, M.; Henriksson, U. Self-diffusion study of counterion complexation in aqueous micellar lithium dodecyl sulfate solutions. J. Colloid. Interface Sci. 1992, 150, 281–284. [Google Scholar] [CrossRef]
- Tanner, J.E. Use of stimulated echo in NMR diffusion studies. J. Chem. Phys. 1970, 52, 2523–2526. [Google Scholar] [CrossRef]
- Spěváček, J.; Suchopárek, M.; Al-Alawi, S. Characterization of the stereochemical structure of poly(acrylic acid) by one- and two-dimensional 13C-1H nuclear magnetic resonance spectra. Polymer 1995, 36, 4125–4130. [Google Scholar] [CrossRef]
- Hikichi, K.; Ikura, M.; Yasuda, M. Two-Dimensional 1H and 13C Nuclear Magnetic Resonance Studies of Poly(acrylamide). Polym. J. 1988, 20, 851–859. [Google Scholar] [CrossRef] [Green Version]







| Block Copolymer | PAAm/g·mol−1 | PAA/g·mol−1 | PDI a |
|---|---|---|---|
| PAAm133-b-PAA49 | 9415 | 3500 | 2.1 b |
| PAAm432-b-PAA70 | 30,680 | 5000 | 1.6 c |
| Species | D/× 10−10 m2.s−1 | Peak Position/ppm |
|---|---|---|
| C12TA+ a | 4.86 | 3.1 |
| C16TA+ b | 4.14 | 3.1 |
| PAAm133-b-PAA49 c | 0.66 | 1.7 |
| PAAm432-b-PAA70 d | 0.31 | 1.7 |
| BCPCS | Species | D/m2.s−1 |
|---|---|---|
| C12S | C12TA+ PAAm133-b-PA49 HMDSO | 1.43 × 10−10 2.33 × 10−12 2.12 × 10−12 |
| C12L | C12TA+ PAAm432-b-PA70 HMDSO | 1.38 × 10−10 2.10 × 10−12 1.90 × 10−12 |
| C16S | C16TA+ PAAm133-b-PA49 HMDSO | 1.32 × 10−10 2.35 × 10−12 2.18 × 10−12 |
| Sample | Dsurf × 10−10/m2·s−1 | Dpol × 10−12/m2·s−1 | RH (DLS)/nm c | RH (Dpol)/nm d |
|---|---|---|---|---|
| C12S a | 1.60 | 3.00 | 120 | 131 |
| C12L a | 1.60 | 2.40 | 130 | 164 |
| C12S b | 1.40 | 7.00 | 50 | 56 |
| C12L b | 1.70 | 6.30 | 55 | 63 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, G.A.; Loh, W.; Topgaard, D.; Söderman, O.; Piculell, L. Molecular Assembly in Block Copolymer-Surfactant Nanoparticle Dispersions: Information on Molecular Exchange and Apparent Solubility from High-Resolution and PFG NMR. Polymers 2021, 13, 3265. https://doi.org/10.3390/polym13193265
Ferreira GA, Loh W, Topgaard D, Söderman O, Piculell L. Molecular Assembly in Block Copolymer-Surfactant Nanoparticle Dispersions: Information on Molecular Exchange and Apparent Solubility from High-Resolution and PFG NMR. Polymers. 2021; 13(19):3265. https://doi.org/10.3390/polym13193265
Chicago/Turabian StyleFerreira, Guilherme A., Watson Loh, Daniel Topgaard, Olle Söderman, and Lennart Piculell. 2021. "Molecular Assembly in Block Copolymer-Surfactant Nanoparticle Dispersions: Information on Molecular Exchange and Apparent Solubility from High-Resolution and PFG NMR" Polymers 13, no. 19: 3265. https://doi.org/10.3390/polym13193265