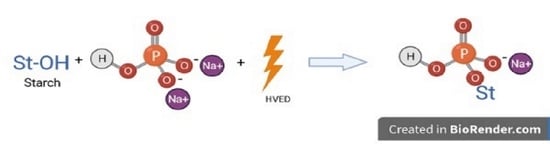
Phosphorylation of Maize Starch Enhanced with High-Voltage Electrical Discharge (HVED) Instead of Thermal Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Phosphorylation of Starch
2.1.1. Phosphorylation with Na2HPO4
2.1.2. Phosphorylation with Na5P3O10
2.2. HVED Treatment of Starch
2.3. Combination of HVED Treatment with Phosphorylation
2.4. Determination of Phosphorus Content
2.5. Determination of Swelling Power and Solubility in Water
2.6. Determination of the Mean Volumetric Diameter
2.7. Determination of Thermal Characteristics
2.8. Rheological Measurements
2.8.1. Determination of the Flow Curves of Pastes
2.8.2. Determination of the Mechanical Spectra of Gels
2.9. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rożnowski, J.; Juszczak, L.; Szwaja, B.; Przetaczek-Rożnowska, I. Effect of Esterification Conditions on the Physicochemical Properties of Phosphorylated Potato Starch. Polymers 2021, 13, 2548. [Google Scholar] [CrossRef]
- Xie, W.; Shao, L. Phosphorylation of corn starch in an ionic liquid. Starch-Stärke 2009, 61, 702–708. [Google Scholar] [CrossRef]
- Ramadan, M.F.; Sitohy, M.Z. Phosphorylated Starches: Preparation, Properties, Functionality, and Techno-Applications. Starch-Stärke 2020, 72, 1900302. [Google Scholar] [CrossRef]
- Muhammad, A.I.; Xiang, Q.; Liao, X.; Liu, D.; Ding, T. Understanding the Impact of Nonthermal Plasma on Food Constituents and Microstructure—A Review. Food Bioprocess Technol. 2018, 11, 463–486. [Google Scholar] [CrossRef]
- Puértolas, E.; Barba, F.J. Electrotechnologies applied to valorization of by-products from food industry: Main findings, energy and economic cost of their industrialization. Food Bioprod. Process. 2016, 100, 172–184. [Google Scholar] [CrossRef]
- Li, Z.; Fan, Y.; Xi, J. Recent advances in high voltage electric discharge extraction of bioactive ingredients from plant materials. Food Chem. 2019, 277, 246–260. [Google Scholar] [CrossRef] [PubMed]
- Jokić, S.; Pavlović, N.; Jozinović, A.; Ačkar, Đ.; Babić, J.; Šubarić, D. High-voltage electric discharge extraction of bioactive compounds from the cocoa bean shell. Chem. Biochem. Eng. Q. 2019, 33, 271–280. [Google Scholar] [CrossRef]
- Gavahian, M.; Cullen, P.J. Cold Plasma as an Emerging Technique for Mycotoxin-Free Food: Efficacy, Mechanisms, and Trends. Food Rev. Int. 2020, 36, 193–214. [Google Scholar] [CrossRef]
- Anpilov, A.M.; Barkhudarov, E.M.; Christofi, N.; Kopev, V.A.; Kossyi, I.A.; Taktakishvili, M.I.; Zadiraka, Y. Pulsed high voltage electric discharge disinfection of microbially contaminated liquids. Lett. Appl. Microbiol. 2002, 35, 90–94. [Google Scholar] [CrossRef] [Green Version]
- Tessier, D.; Oguic, C.; Pinart, J.; Aaron, J.J. Usefulness of a technique based on negative corona discharge for the degradation of selected, condensed PAHs: Application to the oxidation of anthracene and similar structures. Turk. J. Chem. 2001, 25, 157–164. [Google Scholar]
- Grinevich, V.I.M.; Kvitkova, E.Y.; Plastinina, N.A.; Rybkin, V.V. Application of dielectric barrier discharge for waste water purification. Plasma Chem. Plasma Process. 2011, 31, 573–583. [Google Scholar] [CrossRef]
- Grgić, I.; Ačkar, Đ.; Barišić, V.; Vlainić, M.; Knežević, N.; Medverec Knežević, Z. Nonthermal methods for starch modification—A review. J. Food Process. Preserv. 2019, 43, e14242. [Google Scholar] [CrossRef]
- Prasanthi, N.L.; Rao, N.R. Starch Phosphate: A Novel Pharmaceutical Excipient For Tablet Formulation. J. Pharm. Res. 2010, 3, 2919–2923. [Google Scholar]
- Lim, S.; Seib, P.A. Preparation and Pasting Properties of Wheat and Corn Starch Phosphates. Cereal Chem. 1993, 70, 137–144. [Google Scholar]
- Barišić, V.; Jozinović, A.; Flanjak, I.; Šubarić, D.; Babić, J.; Miličević, B.; Doko, K.; Ačkar, Đ. Difficulties with use of cocoa bean shell in food production and high voltage electrical discharge as a possible solution. Sustainability 2020, 12, 3981. [Google Scholar] [CrossRef]
- Kapelko-Żeberska, M.; Zięba, T.; Pietrzak, W.; Gryszkin, A. Effect of citric acid esterification conditions on the properties of the obtained resistant starch. Int. J. Food Sci. Technol. 2016, 51, 1647–1654. [Google Scholar] [CrossRef]
- Gryszkin, A.; Zieba, T.; Kapelko, M.; Buczek, A. Effect of thermal modifications of potato starch on its selected properties. Food Hydrocoll. 2014, 40, 122–127. [Google Scholar] [CrossRef]
- Ziȩba, T.; Szumny, A.; Kapelko, M. Properties of retrograded and acetylated starch preparations: Part 1. Structure, susceptibility to amylase, and pasting characteristics. LWT Food Sci. Technol. 2011, 44, 1314–1320. [Google Scholar] [CrossRef]
- Ziȩba, T.; Juszczak, L.; Gryszkin, A. Properties of retrograded and acetylated starch preparations Part 2. Dynamics of saccharification with amyloglucosidase and rheological properties of resulting pastes and gels. LWT Food Sci. Technol. 2011, 44, 1321–1327. [Google Scholar] [CrossRef]
- Gryszkin, A.; Zieba, T.; Kapelko-Zeberska, M.; Atraszkiewicz, A. Hydrothermal modification of wheat starch part 1. Effect of particle size on the viscosity of formed pastes. J. Cereal Sci. 2016, 68, 46–52. [Google Scholar] [CrossRef]
- Takeda, Y.; Shitaozono, T.; Hizukuri, S. Molecular Structure of Corn Starch. Starch-Stärke 1988, 40, 51–54. [Google Scholar] [CrossRef]
- Du, C.M.; Yan, J.H.; Li, X.D.; Cheron, B.G.; You, X.F.; Chi, Y.; Ni, M.J.; Cen, K.F. Simultaneous removal of polycyclic aromatic hydrocarbons and soot particles from flue gas by gliding arc discharge treatment. Plasma Chem. Plasma Process. 2006, 26, 517–525. [Google Scholar] [CrossRef]
- Malumba, P.; Massaux, C.; Deroanne, C.; Masimango, T.; Béra, F. Influence of drying temperature on functional properties of wet-milled starch granules. Carbohydr. Polym. 2009, 75, 299–306. [Google Scholar] [CrossRef]
- Thirumdas, R.; Kothakota, A.; Annapure, U. Plasma Activated Water (PAW): Chemistry, Physico-Chemical Properties, Applications in Food and Agriculture. Trends Food Sci. Technol. 2018, 80, 93–103. [Google Scholar] [CrossRef]
- Thirumdas, R.; Sarangapani, C.; Annapure, U.S. Cold Plasma: A novel Non-Thermal Technology for Food Processing. Food Biophys. 2015, 10, 1–11. [Google Scholar] [CrossRef]
- Bie, P.; Pu, H.; Zhang, B.; Su, J.; Chen, L.; Li, X. Structural characteristics and rheological properties of plasma-treated starch. Innov. Food Sci. Emerg. Technol. 2016, 34, 196–204. [Google Scholar] [CrossRef]
- Khorram, S.; Zakerhamidi, M.S.; Karimzadeh, Z. Polarity functions’ characterization and the mechanism of starch modification by DC glow discharge plasma. Carbohydr. Polym. 2015, 127, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Andrade, C.T.; Simão, R.A.; Thiré, R.M.S.M.; Achete, C.A. Surface modification of maize starch films by low-pressure glow 1-butene plasma. Carbohydr. Polym. 2005, 61, 407–413. [Google Scholar] [CrossRef]
- Thirumdas, R.; Kadam, D.; Annapure, U.S. Cold Plasma: An Alternative Technology for the Starch Modification. Food Biophys. 2017, 12, 129–139. [Google Scholar] [CrossRef]
- Ascheri, D.P.R.; Pereira, L.D.; Bastos, S.M.C. Chemical, morphological, rheological and thermal properties of Solanum lycocarpum phosphorylated starches. Rev. Ceres 2014, 61, 458–466. [Google Scholar] [CrossRef] [Green Version]
- Wongsagonsup, R.; Deeyai, P.; Chaiwat, W.; Fuongfuchat, A.; Varavinit, S.; Dangtip, S.; Suphantharika, M. Modification of tapioca starch by non-chemical route using jet atmospheric argon plasma. Carbohydr. Polym. 2014, 102, 790–798. [Google Scholar] [CrossRef] [PubMed]
- Hornung, P.S.; Barbi, R.C.T.; Teixeira, G.L.; Avila, S.; da Silva, F.L.A.; Lazzarotto, M.; Silveira, J.L.M.; Beta, T.; Ribani, R.H. Brazilian Amazon white yam (Dioscorea sp.) starch: Impact on functional properties due to chemical and physical modifications processes. J. Therm. Anal. Calorim. 2018, 134, 2075–2088. [Google Scholar] [CrossRef]
- Park, S.; Chung, M.G.; Yoo, B. Effect of octenylsuccinylation on rheological properties of corn starch pastes. Starch-Stärke 2004, 56, 399–406. [Google Scholar] [CrossRef]
- Heo, H.; Lee, Y.K.; Chang, Y.H. Rheological, pasting, and structural properties of potato starch by cross-linking. Int. J. Food Prop. 2017, 20, 2138–2150. [Google Scholar] [CrossRef]

| Treatment | Phosphorus Content (g/kg) |
|---|---|
| native | 0.144 ± 0.002a |
| HVED30 | 0.137 ± 0.003a |
| HVED30_T | 0.139 ± 0.005a |
| MONOP | 0.187 ± 0.003a |
| MONOP_T | 0.434 ± 0.011a,b |
| HVED10_MONOP | 3.831 ± 0.014c |
| HVED30_MONOP | 3.411 ± 0.009c |
| MONOP_HVED10 | 2.781 ± 0.012c |
| MONOP_HVED30 | 3.892 ± 0.021c |
| TRIP | 0.168 ± 0.009a |
| TRIP_T | 0.855 ± 0.011b |
| HVED10_TRIP | 0.749 ± 0.014b |
| HVED30_TRIP | 0.628 ± 0.008b |
| TRIP_HVED10 | 0.706 ± 0.009b |
| TRIP_HVED30 | 0.757 ± 0.012b |
| Treatment | DSC Gelatinisation Parameters A | Mean Volumetric Diameter (µm) B | ||
|---|---|---|---|---|
| to (°C) B | tc (°C) B | ΔH (J/g) B | ||
| native | 64.36 ± 0.04f | 75.17 ± 0.02f | 12.28 ± 0.37b | 14.55 ± 0.01a |
| HVED30 | 64.17 ± 0.09f | 74.79 ± 0.05e | 12.90 ± 0.43d–f | 21.10 ± 0.51i |
| HVED30_T | 62.84 ± 0.04c,d | 74.04 ± 0.08a | 12.56 ± 0.11b–e | 19.51 ± 0.03h |
| MONOP | 65.23 ± 0.60g | 75.00 ± 0.08f | 13.27 ± 0.59f–h | 17.58 ± 0.03e |
| MONOP_T | 62.61 ± 0.04b,c | 73.94 ± 0.16a | 12.92 ± 0.05d–f | 17.61 ± 0.02e |
| HVED10_MONOP | 65.64 ± 0.10h | 76.20 ± 0.08j | 13.44 ± 0.35g,h | 17.20 ± 0.13d |
| HVED30_MONOP | 65.30 ± 0.19g | 75.90 ± 0.01h | 12.83 ± 0.01c–f | 17.50 ± 0.03e |
| MONOP_HVED10 | 65.13 ± 0.01g | 75.70 ± 0.26g | 12.26 ± 0.18b | 17.57 ± 0.02e |
| MONOP_HVED30 | 65.62 ± 0.12h | 76.49 ± 0.11i | 12.48 ± 0.07b–d | 16.93 ± 0.02c |
| TRIP | 64.24 ± 0.05f | 74.74 ± 0.06d,e | 13.58 ± 0.03h | 16.67 ± 0.04b |
| TRIP_T | 61.18 ± 0.08a | 74.50 ± 0.01b,c | 12.35 ± 0.22b,c | 16.73 ± 0.01b,c |
| HVED10_TRIP | 62.98 ± 0.10d,e | 74.75 ± 0.04d,e | 12.45 ± 0.50b–d | 21.50 ± 0.13j |
| HVED30_TRIP | 63.16 ± 0.03e | 74.36 ± 0.07b | 13.02 ± 0.17e–g | 18.30 ± 0.03g |
| TRIP_HVED10 | 63.02 ± 0.25d,e | 74.56 ± 0.25c,d | 11.76 ± 0.33a | 17.94 ± 0.03f |
| TRIP_HVED30 | 62.52 ± 0.03b | 74.52 ± 0.05b,c | 13.02 ± 0.06e–g | 21.10 ± 0.17i |
| Ostwald de Waele | |||
|---|---|---|---|
| Treatment | K (Pasn) | n | R2 |
| native | 2.331 ± 0.052a | 0.483 ± 0.003d–f | 0.9878 |
| HVED30 | 3.151 ± 0.383a | 0.450 ± 0.012c | 0.9823 |
| HVED30_T | 2.492 ± 0.105a | 0.468 ± 0.007f,g | 0.9819 |
| MONOP | 3.261 ± 0.657a | 0.453 ± 0.033c | 0.9947 |
| MONOP_T | 5.538 ± 0.577b,c | 0.411 ± 0.014g | 0.9797 |
| HVED10_MONOP | 5.404 ± 1.857b | 0.410 ± 0.061a,b | 0.9745 |
| HVED30_MONOP | 6.572 ± 1.054b–d | 0.369 ± 0.021c–e | 0.9699 |
| MONOP_HVED10 | 5.297 ± 0.521b | 0.403 ± 0.011a | 0.9784 |
| MONOP_HVED30 | 6.828 ± 1.156c,d | 0.359 ± 0.023b,c | 0.9648 |
| TRIP | 2.623 ± 0.135a | 0.488 ± 0.013a,b | 0.9869 |
| TRIP_T | 11.737 ± 1.181e | 0.371 ± 0.013f,g | 0.9718 |
| HVED10_TRIP | 7.094 ± 0.584d | 0.421 ± 0.007c–e | 0.9870 |
| HVED30_TRIP | 6.565 ± 0.902b–d | 0.426 ± 0.025b,c | 0.9888 |
| TRIP_HVED10 | 7.798 ± 0.412d | 0.401 ± 0.013c,d | 0.9839 |
| TRIP_HVED30 | 7.672 ± 0.606d | 0.415 ± 0.011e,f | 0.9873 |
| Herschel Bulkly | |||
| Treatment | τ0 (Pa) | KHB (Pasn) | R2 |
| native | 8.057 ± 0.197a-c | 0.335 ± 0.007a,b | 0.9990 |
| HVED30 | 10.586 ± 1.091c | 0.314 ± 0.018a,b | 0.9980 |
| HVED30_T | 9.083 ± 0.380b,c | 0.236 ± 0.015a | 0.9975 |
| MONOP | 7.106 ± 1.238a,b | 0.977 ± 0.073e | 0.9996 |
| MONOP_T | 15.867 ± 1.505d,e | 0.574 ± 0.035c,d | 0.9960 |
| HVED10_MONOP | 16.250 ± 4.444d,e | 0.370 ± 0.105a,b | 0.9967 |
| HVED30_MONOP | 18.017 ± 2.209d-f | 0.368 ± 0.044a,b | 0.9942 |
| MONOP_HVED10 | 15.123 ± 1.667d | 0.481 ± 0.064b,c | 0.9959 |
| MONOP_HVED30 | 18.850 ± 2.494e,f | 0.290 ± 0.055a | 0.9931 |
| TRIP | 5.178 ± 0.532a | 1.101 ± 0.182e,f | 0.9998 |
| TRIP_T | 32.323 ± 2.776g | 0.711 ± 0.107d | 0.9982 |
| HVED10_TRIP | 18.650 ± 1.530e,f | 1.133 ± 0.039e,f | 0.9986 |
| HVED30_TRIP | 16.737 ± 2.867d,e | 1.187 ± 0.199f | 0.9991 |
| TRIP_HVED10 | 20.517 ± 1.287f | 0.970 ± 0.155e | 0.9985 |
| TRIP_HVED30 | 19.697 ± 2.011f | 1.224 ± 0.139f | 0.9988 |
| Casson | |||
| Treatment | τ0C (Pa) | ηC (Pas) | R2 |
| native | 5.805 ± 0.111a | 0.047 ± 0.000a-c | 0.9985 |
| HVED30 | 7.489 ± 0.779a | 0.047 ± 0.002a-c | 0.9969 |
| HVED30_T | 6.080 ± 0.201a | 0.044 ± 0.001a | 0.9962 |
| MONOP | 7.599 ± 1.213a | 0.050 ± 0.005b-d | 0.9990 |
| MONOP_T | 12.337 ± 1.024b | 0.059 ± 0.002f | 0.9952 |
| HVED10_MONOP | 11.755 ±3.123b | 0.055 ± 0.009d,e | 0.9945 |
| HVED30_MONOP | 13.477 ± 1.599b | 0.048 ± 0.002a-c | 0.9924 |
| MONOP_HVED10 | 11.590 ± 0.941b | 0.053 ± 0.001c–e | 0.9951 |
| MONOP_HVED30 | 13.740 ± 1.695b,c | 0.046 ± 0.002a,b | 0.9903 |
| TRIP | 6.425 ± 0.217a | 0.056 ± 0.003d,e | 0.9985 |
| TRIP_T | 24.443 ± 1.825e | 0.086 ± 0.002g | 0.9960 |
| HVED10_TRIP | 16.080 ± 1.156d | 0.083 ± 0.003g | 0.9982 |
| HVED30_TRIP | 14.907 ± 1.597c,d | 0.081 ± 0.007f,g | 0.9987 |
| TRIP_HVED10 | 17.067 ± 0.570d | 0.076 ± 0.005f | 0.9980 |
| TRIP_HVED30 | 17.170 ± 1.065d | 0.085 ± 0.002g | 0.9984 |
| Treatment | G′ (Pa) | G″ (Pa) | G″/G′ |
|---|---|---|---|
| native | 33.90 ± 0.69a,b | 4.64 ± 0.04b | 0.14 ± 0.00a |
| HVED30 | 40.04 ± 3.14b,c | 5.23 ± 0.36c | 0.13 ± 0.00a |
| HVED30_T | 31.49 ± 2.06a | 4.05 ± 0.10a | 0.13 ± 0.01a |
| MONOP | 29.23 ± 9.60a | 5.83 ± 0.29d | 0.21 ± 0.07b |
| MONOP_T | 41.52 ± 2.41b,c | 6.62 ± 0.20e | 0.16 ± 0.00a |
| HVED10_MONOP | 45.66 ± 0.78c | 6.14 ± 0.18d,e | 0.13 ± 0.01a |
| HVED30_MONOP | 43.04 ± 2.39c | 6.00 ± 0.14d | 0.14 ± 0.01a |
| MONOP_HVED10 | 41.14 ± 2.09b,c | 5.99 ± 0.20d | 0.15 ± 0.00a |
| MONOP_HVED30 | 43.77 ± 3.16c | 5.91 ± 0.14d | 0.14 ± 0.01a |
| TRIP | 26.56 ± 7.68a | 6.00 ± 0.26d | 0.24 ± 0.05b |
| TRIP_T | 57.45 ± 1.06d,e | 9.03 ± 0.21f | 0.16 ± 0.00a |
| HVED10_TRIP | 69.27 ± 1.26f | 9.75 ± 0.10g | 0.14 ± 0.00a |
| HVED30_TRIP | 61.90 ± 10.88d-f | 8.99 ± 0.80f | 0.15 ± 0.01a |
| TRIP_HVED10 | 56.92 ± 3.66d | 8.79 ± 0.49f | 0.15 ± 0.00a |
| TRIP_HVED30 | 64.52 ± 1.96e,f | 9.62 ± 0.12g | 0.15 ± 0.00a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gryszkin, A.; Grec, M.; Ačkar, Đ.; Zięba, T.; Jozinović, A.; Šubarić, D.; Miličević, B.; Blažić, M.; Babić, J. Phosphorylation of Maize Starch Enhanced with High-Voltage Electrical Discharge (HVED) Instead of Thermal Treatment. Polymers 2021, 13, 3231. https://doi.org/10.3390/polym13193231
Gryszkin A, Grec M, Ačkar Đ, Zięba T, Jozinović A, Šubarić D, Miličević B, Blažić M, Babić J. Phosphorylation of Maize Starch Enhanced with High-Voltage Electrical Discharge (HVED) Instead of Thermal Treatment. Polymers. 2021; 13(19):3231. https://doi.org/10.3390/polym13193231
Chicago/Turabian StyleGryszkin, Artur, Marijana Grec, Đurđica Ačkar, Tomasz Zięba, Antun Jozinović, Drago Šubarić, Borislav Miličević, Marijana Blažić, and Jurislav Babić. 2021. "Phosphorylation of Maize Starch Enhanced with High-Voltage Electrical Discharge (HVED) Instead of Thermal Treatment" Polymers 13, no. 19: 3231. https://doi.org/10.3390/polym13193231