Green Synthesis of Stable Nanocomposites Containing Copper Nanoparticles Incorporated in Poly-N-vinylimidazole
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Poly-N-vinylimidazole
2.3. Synthesis of Nanocomposites with Copper Nanoparticles
2.4. Characterization
3. Results and Discussion
3.1. Polymer of N-vinylimidazole
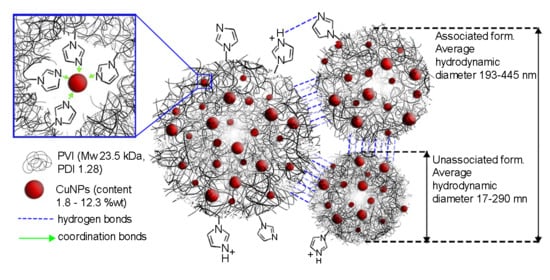
3.2. Synthesis and Characterization of Polymeric CuNPs Nanocomposites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ivanchev, S.S.; Ozerin, A.N. Nanostructures in polymer systems. Polym. Sci. Ser. B 2006, 48, 213–225. [Google Scholar] [CrossRef]
- Pomogailo, A.D.; Rosenberg, A.S.; Uflyand, I.E. Metal Nanoparticles in Polymers; Khimiya: Moscow, Russia, 2000. [Google Scholar]
- Salata, O. Applications of nanoparticles in biology and medicine. J. Nanobiotechnol. 2004, 2, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.H.; Gulumian, M.; Faustman, E.M.; Workman, T.; Jeon, K.; Yu, I.J. Blood Biochemical and Hematological Study after Subacute Intravenous Injection of Gold and Silver Nanoparticles and Coadministered Gold and Silver Nanoparticles of Similar Sizes. BioMed Res. Int. 2018, 2018, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shurygina, I.A.; Prozorova, G.F.; Trukhan, I.S.; Korzhova, S.A.; Fadeeva, T.V.; Pozdnyakov, A.S.; Dremina, N.N.; Emel’Yanov, A.I.; Kuznetsova, N.P.; Shurygin, M.G. NonToxic Silver/Poly-1-Vinyl-1,2,4-Triazole Nanocomposite Materials with Antibacterial Activity. Nanomaterials 2020, 10, 1477. [Google Scholar] [CrossRef] [PubMed]
- Haseeb, M.T.; Hussain, M.A.; Abbas, K.; Youssif, B.G.; Bashir, S.; Yuk, S.H.; Bukhari, S.N.A. Linseed hydrogel-mediated green synthesis of silver nanoparticles for antimicrobial and wound-dressing applications. Int. J. Nanomed. 2017, ume 12, 2845–2855. [Google Scholar] [CrossRef] [Green Version]
- Prozorova, G.F.; Korzhova, S.A.; Pozdnyakov, A.; Emel´yanov, A.I.; Ermakova, T.G.; Dubrovina, V.I. Immunomodulatory properties of silver-containing nanocomposite on the basis of polyvinyltriazole. Russ. Chem. Bull. 2015, 64, 1437–1439. [Google Scholar] [CrossRef]
- Volkov, V.V.; Kravchenko, T.A.; Roldughin, V.I. Metal nanoparticles in catalytic polymer membranes and ion-exchange systems for advanced purification of water from molecular oxygen. Russ. Chem. Rev. 2013, 82, 465–482. [Google Scholar] [CrossRef]
- Zezin, A.A. Synthesis of Metal-Polymer Complexes and Functional Nanostructures in Films and Coatings of Interpolyelectrolyte Complexes. Polym. Sci. Ser. A 2019, 61, 754–764. [Google Scholar] [CrossRef]
- Johnston, R.L. Metal Nanoparticles and Nanoalloys. In Frontiers of Nanoscience; Elsevier: Amsterdam, The Netherlands, 2012; pp. 1–42. [Google Scholar] [CrossRef]
- Hoover, N.N.; Auten, B.J.; Chandler, B. Tuning Supported Catalyst Reactivity with Dendrimer-Templated Pt−Cu Nanoparticles. J. Phys. Chem. B 2006, 110, 8606–8612. [Google Scholar] [CrossRef] [Green Version]
- Weir, E.; Lawlor, A.; Whelan, A.; Regan, F. The use of nanoparticles in anti-microbial materials and their characterization. Analyst 2008, 133, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Zezina, E.A.; Emel’Yanov, A.I.; Pozdnyakov, A.S.; Prozorova, G.F.; Abramchuk, S.S.; Feldman, V.I.; Zezin, A.A. Radiation-induced synthesis of copper nanostructures in the films of interpolymer complexes. Radiat. Phys. Chem. 2019, 158, 115–121. [Google Scholar] [CrossRef]
- Yoshida, K.; González-Arellano, C.; Luque, R.; Gai, P.L. Efficient hydrogenation of carbonyl compounds using low-loaded supported copper nanoparticles under microwave irradiation. Appl. Catal. A Gen. 2010, 379, 38–44. [Google Scholar] [CrossRef]
- Shih, Z.-Y.; Periasamy, A.P.; Hsu, P.-C.; Chang, H.-T. Synthesis and catalysis of copper sulfide/carbon nanodots for oxygen reduction in direct methanol fuel cells. Appl. Catal. B Environ. 2013, 132-133, 363–369. [Google Scholar] [CrossRef]
- Allen, S.E.; Walvoord, R.; Padilla-Salinas, R.; Kozlowski, M.C. Aerobic Copper-Catalyzed Organic Reactions. Chem. Rev. 2013, 113, 6234–6458. [Google Scholar] [CrossRef] [Green Version]
- Pawar, R.C.; Choi, D.-H.; Lee, J.-S.; Lee, C.S. Formation of polar surfaces in microstructured ZnO by doping with Cu and applications in photocatalysis using visible light. Mater. Chem. Phys. 2015, 151, 167–180. [Google Scholar] [CrossRef]
- Ahn, Y.; Jeong, Y.; Lee, D.; Lee, Y. Copper Nanowire–Graphene Core–Shell Nanostructure for Highly Stable Transparent Conducting Electrodes. ACS Nano 2015, 9, 3125–3133. [Google Scholar] [CrossRef] [PubMed]
- Ressler, T.; Kniep, B.L.; Kasatkin, I.; Schlögl, R. The Microstructure of Copper Zinc Oxide Catalysts: Bridging the Materials Gap. Angew. Chem. Int. Ed. 2005, 44, 4704–4707. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Das, A.; Zhang, X.; Schatz, G.C.; Sligar, S.G.; Van Duyne, R.P. Resonance Surface Plasmon Spectroscopy: Low Molecular Weight Substrate Binding to Cytochrome P450. J. Am. Chem. Soc. 2006, 128, 11004–11005. [Google Scholar] [CrossRef]
- Rakhmetova, A.A.; Alekseeva, T.P.; Bogoslovskaya, O.A.; Leipunskii, I.O.; Ol’Khovskaya, I.P.; Zhigach, A.N.; Glushchenko, N.N. Wound-healing properties of copper nanoparticles as a function of physicochemical parameters. Nanotechnol. Russ. 2010, 5, 271–276. [Google Scholar] [CrossRef]
- Chatterjee, A.K.; Sarkar, R.K.; Chattopadhyay, A.P.; Aich, P.; Chakraborty, R.; Basu, T. A simple robust method for synthesis of metallic copper nanoparticles of high antibacterial potency againstE. coli. Nanotechnology 2012, 23, 085103. [Google Scholar] [CrossRef]
- Ramyadevi, J.; Jeyasubramanian, K.; Marikani, A.; Rajakumar, G.; Rahuman, A.A. Synthesis and antimicrobial activity of copper nanoparticles. Mater. Lett. 2012, 71, 114–116. [Google Scholar] [CrossRef]
- Glushchenko, N.N.; Bogoslovskaya, O.A.; Ol’khovskaya, I.P. Physicochemical regularities in biological action of highly dispersed powders of metals. Russ. J. Phys. Chem. 2002, 21, 79–85. [Google Scholar]
- Malandrakis, A.A.; Kavroulakis, N.; Chrysikopoulos, C. Synergy between Cu-NPs and fungicides against Botrytis cinerea. Sci. Total. Environ. 2019, 703, 135557. [Google Scholar] [CrossRef]
- Ostaeva, G.Y.; Selishcheva, E.D.; Papisov, I.M. Competition between polyelectrolyte macromolecules and amphiphilic polymer micelles in interaction with copper nanoparticles. Polym. Sci. Ser. B 2007, 49, 10–14. [Google Scholar] [CrossRef]
- Abdel-Aziz, M.; Morsi, S.; Salama, D.; Elwahed, M.S.A.; Shaaban, M.; Youssef, A. Preparation and characterization of chitosan/polyacrylic acid/copper nanocomposites and their impact on onion production. Int. J. Biol. Macromol. 2018, 123, 856–865. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Sun, B.; Huang, Y.; Chen, C.; Sun, D. Facile synthesis of Cu nanoparticles encapsulated into carbonized bacterial cellulose with excellent oxidation resistance and stability. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 590, 124462. [Google Scholar] [CrossRef]
- Granata, G.; Onoguchi, A.; Tokoro, C. Preparation of copper nanoparticles for metal-metal bonding by aqueous reduction with d-glucose and PVP. Chem. Eng. Sci. 2019, 209, 115210. [Google Scholar] [CrossRef]
- Zhou, Y.; Yu, S.; Niu, H.; Liu, F. Synergistic Improvement in Thermal Conductivity of Polyimide Nanocomposite Films Using Boron Nitride Coated Copper Nanoparticles and Nanowires. Polymers 2018, 10, 1412. [Google Scholar] [CrossRef] [Green Version]
- Trofimova, O.M.; Pozdnyakov, A.S.; Emel’Yanov, A.I.; Kuznetsova, N.P.; Ermakova, T.G.; Bolgova, Y.I.; Albanov, A.I.; Borodina, T.N.; Smirnov, V.I.; Prozorova, G.F. A Polymer Nanocomposite with CuNP Stabilized by 1-Vinyl-1,2,4-triazole and Acrylonitrile Copolymer. Synlett 2015, 27, 900–904. [Google Scholar] [CrossRef]
- Feldman, V.; Zezin, A.A.; Abramchuk, S.S.; Zezina, E. X-ray Induced Formation of Metal Nanoparticles from Interpolyelectrolyte Complexes with Copper and Silver Ions: The Radiation-Chemical Contrast. J. Phys. Chem. C 2013, 117, 7286–7293. [Google Scholar] [CrossRef]
- Yuan, S.; Pehkonen, S.; Liang, B.; Ting, Y.; Neoh, K.; Kang, E. Poly(1-vinylimidazole) formation on copper surfaces via surface-initiated graft polymerization for corrosion protection. Corros. Sci. 2010, 52, 1958–1968. [Google Scholar] [CrossRef]
- Nakabayashi, K.; Mori, H. Recent progress in controlled radical polymerization of N-vinyl monomers. Eur. Polym. J. 2013, 49, 2808–2838. [Google Scholar] [CrossRef] [Green Version]
- Selivanova, A.V.; Tyurin, V.S.; Beletskaya, I.P. Palladium Nanoparticles Supported on Poly(N-vinyl-imidazole-co-N-vinylcaprolactam) as an Effective Recyclable Catalyst for the Suzuki Reaction. ChemPlusChem 2014, 79, 1278–1283. [Google Scholar] [CrossRef]
- Dağaş, D.E.; Danelyan, G.V.; Ghaffarlou, M.; Zezina, E.; Abramchuk, S.S.; Feldman, V.I.; Güven, O.; Zezin, A.A. Generation of spatially ordered metal–polymer nanostructures in the irradiated dispersions of poly(acrylic acid)–poly(vinylimidazole)–Cu2+ complexes. Colloid Polym. Sci. 2020, 298, 193–202. [Google Scholar] [CrossRef]
- Isikli, S.; Tuncagil, S.; Bozkurt, A.; Toppare, L. Immobilization of Invertase in a Novel Proton Conducting Poly(vinylphosphonic acid—poly(1-vinylimidazole) Network. J. Macromol. Sci. Part A 2010, 47, 639–646. [Google Scholar] [CrossRef]
- Asayama, S.; Nishinohara, S.; Kawakami, H. Zinc-Chelated Poly(1-vinylimidazole) and a Carbohydrate Ligand Polycation Form DNA Ternary Complexes for Gene Delivery. Bioconjugate Chem. 2011, 22, 1864–1868. [Google Scholar] [CrossRef]
- Henrichs, P.M.; Whitlock, L.R.; Sochor, A.R.; Tan, J.S. Conformational Behavior of Poly(N-vinylimidazole). Potentiometric Titration, Viscosity, and Proton Nuclear Magenetic Resonance Studies. Macromolecules 1980, 13, 1375–1381. [Google Scholar] [CrossRef]
- Dambatta, B.; Ebdon, J.; Huckerby, T. Unusual influences of temperature and medium on the tacticity of radically polymerised poly(n-vinyl imidazole). Eur. Polym. J. 1984, 20, 645–652. [Google Scholar] [CrossRef]
- Bărboiu, V.; Streba, E.; Holerca, M.N.; Luca, C. Reactions on Polymers with Amine Groups. II. Reactions of Poly(N-Vinylimidazole) and Its Model Compound with Unsaturated Carboxylic Acids. J. Macromol. Sci. Part A 1995, 32, 1385–1396. [Google Scholar] [CrossRef]
- Fathima, J.B.; Pugazhendhi, A.; Oves, M.; Venis, R. Synthesis of eco-friendly copper nanoparticles for augmentation of catalytic degradation of organic dyes. J. Mol. Liq. 2018, 260, 1–8. [Google Scholar] [CrossRef]
- Xiong, J.; Wang, Y.; Xue, Q.; Wu, X. Synthesis of highly stable dispersions of nanosized copper particles using l-ascorbic acid. Green Chem. 2011, 13, 900–904. [Google Scholar] [CrossRef]
- Cheng, X.; Zhang, X.; Yin, H.; Wang, A.; Xu, Y. Modifier effects on chemical reduction synthesis of nanostructured copper. Appl. Surf. Sci. 2006, 253, 2727–2732. [Google Scholar] [CrossRef]
- Xie, S.-Y.; Ma, Z.-J.; Wang, C.-F.; Lin, S.-C.; Jiang, Z.-Y.; Huang, R.-B.; Zheng, L.-S. Preparation and self-assembly of copper nanoparticles via discharge of copper rod electrodes in a surfactant solution: A combination of physical and chemical processes. J. Solid State Chem. 2004, 177, 3743–3747. [Google Scholar] [CrossRef]
- Shaulina, L.P.; Skushnikova, A.I.; Domnina, E.S.; Pavlova, A.L.; Golentovskaya, I.P. Sorption of noble-metal ions by vinylimidazole polymers cross-linked with acrylic acid. J. Appl. Chem. 1991, 182–184. [Google Scholar]
- Fodor, C.; Bozi, J.; Blazsó, M.; Iván, B. Thermal Behavior, Stability, and Decomposition Mechanism of Poly(N-vinylimidazole). Macromolecules 2012, 45, 8953–8960. [Google Scholar] [CrossRef]
- Goswami, S.; Dutta, A. Measurement of ionic conductivity of poly (N-vinylimidazole) and its fluoroborate salt in solid state. Ionics 2011, 17, 627–632. [Google Scholar] [CrossRef]
- Lippert, J.L.; Robertson, J.A.; Havens, J.R.; Tan, J.S. Structural studies of poly(N-vinylimidazole) complexes by infrared and Raman spectroscopy. Macromolecules 1985, 18, 63–67. [Google Scholar] [CrossRef]
- Pekel, N.; Güven, O. Investigation of complex formation between poly(N-vinyl imidazole) and various metal ions using the molar ratio method. Colloid Polym. Sci. 1999, 277, 570–573. [Google Scholar] [CrossRef]
- Garrido, L.V.Q.; Gonçalves, J.; Rocha, J.C.; Bastos, E.L.; Toma, H.E.; Zamarion, V.M. Intriguing Plasmonic and Fluorescence Duality in Copper Nanoparticles. Plasmonics 2020, 15, 1213–1219. [Google Scholar] [CrossRef]
- Aguilar, M.; Esparza, R.; Rosas, G. Synthesis of Cu nanoparticles by chemical reduction method. Trans. Nonferrous Met. Soc. China 2019, 29, 1510–1515. [Google Scholar] [CrossRef]
- Pozdnyakov, A.; Ivanova, A.; Emel’Yanov, A.; Bolgova, Y.I.; Trofimova, O.; Prozorova, G. Water-soluble stable polymer nanocomposites with AuNPs based on the functional poly(1-vinyl-1,2,4-triazole-co-N-vinylpyrrolidone). J. Organomet. Chem. 2020, 922, 121352. [Google Scholar] [CrossRef]
- Annenkov, V.V.; Danilovtseva, E.N.; Smirnov, V.I.; Maksimova, M.A. New water-soluble imidazole-containing polymers. Polym. Sci. Ser. B 2005, 47, 201–205. [Google Scholar]
- Annenkov, V.V.; Aseyev, V.; Zelinskiy, S.N.; Danilovtseva, E.N. Imidazole-phosphate polymers: Acid-base properties, association with oligonucleotides and oligosilicates. J. Mol. Liq. 2021, 329, 115598. [Google Scholar] [CrossRef]














| Nanocomposite | PVI:Cu(II), mol | Yield, % | Cu Content, %wt | λmax, nm | Nanoparticle Size, nm | Average Hydrodynamic Diameter, nm | |
|---|---|---|---|---|---|---|---|
| Water | Aqueous Salt Solution | ||||||
| 1 | 40:1 | 85.6 | 1.8 | 556 | 2–8 | 193 | 17 |
| 2 | 20:1 | 83.1 | 3.5 | 557 | 2–10 | 269 | 40 |
| 3 | 10:1 | 85.2 | 6.7 | 535 | 2–12 | 341 | 110 |
| 4 | 5:1 | 84.5 | 12.3 | 539 | 6–20 | 445 | 290 |
| Nanocomposite | Dn, nm | Dw, nm | PDI |
|---|---|---|---|
| 1 | 4.34 | 4.80 | 1.11 |
| 2 | 5.31 | 6.39 | 1.21 |
| 3 | 4.66 | 6.88 | 1.48 |
| 4 | 12.67 | 17.67 | 1.39 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pozdnyakov, A.S.; Emel’yanov, A.I.; Korzhova, S.A.; Kuznetsova, N.P.; Bolgova, Y.I.; Trofimova, O.M.; Semenova, T.A.; Prozorova, G.F. Green Synthesis of Stable Nanocomposites Containing Copper Nanoparticles Incorporated in Poly-N-vinylimidazole. Polymers 2021, 13, 3212. https://doi.org/10.3390/polym13193212
Pozdnyakov AS, Emel’yanov AI, Korzhova SA, Kuznetsova NP, Bolgova YI, Trofimova OM, Semenova TA, Prozorova GF. Green Synthesis of Stable Nanocomposites Containing Copper Nanoparticles Incorporated in Poly-N-vinylimidazole. Polymers. 2021; 13(19):3212. https://doi.org/10.3390/polym13193212
Chicago/Turabian StylePozdnyakov, Alexander S., Artem I. Emel’yanov, Svetlana A. Korzhova, Nadezhda P. Kuznetsova, Yuliya I. Bolgova, Olga M. Trofimova, Tatyana A. Semenova, and Galina F. Prozorova. 2021. "Green Synthesis of Stable Nanocomposites Containing Copper Nanoparticles Incorporated in Poly-N-vinylimidazole" Polymers 13, no. 19: 3212. https://doi.org/10.3390/polym13193212