Water-Soluble Visible Light Sensitive Photoinitiating System Based on Charge Transfer Complexes for the 3D Printing of Hydrogels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Compounds
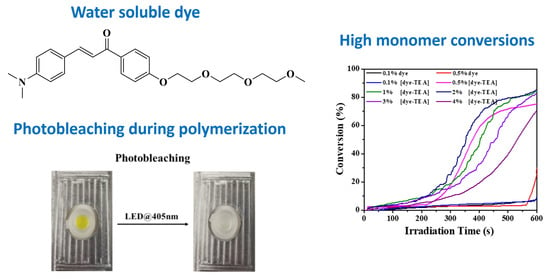
2.1.1. Synthesis of the Water Soluble Chalcone
2.1.2. Synthesis of 2-(2-(2-Methoxyethoxy)ethoxy)ethyl Methanesulfonate
2.1.3. Synthesis of 1-(4-(2-(2-(2-Methoxyethoxy)ethoxy)ethoxy)phenyl)ethan-1-one
2.1.4. Synthesis of 3-(4-(Dimethylamino)phenyl)-1-(4-(2-(2-(2-methoxyethoxy)ethoxy)ethoxy) phenyl)prop-2-en-1-one
2.2. Photopolymerization Experiments
2.3. Characterization
2.4. Direct Laser Write (DLW) Experiment
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Censi, R.; Schuurman, W.; Malda, J.; di Dato, G.; Burgisser, P.E.; Dhert, W.J.A.; van Nostrum, C.F.; di Martino, P.; Vermonden, T.; Hennink, W.E. A Printable Photopolymerizable Thermosensitive p(HPMAm-lactate)-PEG Hydrogel for Tissue Engineering. Adv. Funct. Mater. 2011, 21, 1833–1842. [Google Scholar] [CrossRef]
- Pereira, R.F.; Bártolo, P.J. Photopolymerizable Hydrogels in Regenerative Medicine and Drug Delivery; Future Medicine Ltd.: London, UK, 2014. [Google Scholar]
- Fouassier, J.P.; Lalevée, J. Photoinitiators for Polymer Synthesis. Scope, Reactivity, and Efficiency; Wiley-VCH: Weinheim, Germany, 2012. [Google Scholar]
- Temel, G.; Aydogan, B.; Arsu, N.; Yagci, Y. Polymerized Ionic Liquids: The Effect of Random Copolymer Composition on Ion Conduction. Macromolecules 2009, 42, 6098–6109. [Google Scholar] [CrossRef]
- Dietliker, D. Water-soluble Photoinitiators: Present and Future. RSC Polym. Chem. Ser. 2018, 13, 358–430. [Google Scholar]
- Barker, P.; Guthrie, J.T.; Davis, M.J.; Godfrey, A.A.; Green, P.N. Sensitized photoinitiated grafting of N-vinyl-2-prrolidone (NVP) to woolen substrates. J. Appl. Polym. Sci. 1981, 26, 521–527. [Google Scholar] [CrossRef]
- Benedikt, S.; Wang, J.P.; Markovic, M.; Moszner, N.; Dietliker, K.; Ovsianikov, A.; Grützmacher, H.; Liska, R. Highly efficient water-soluble visible light photoinitiators. J. Polym. Sci. A Polym. Chem. 2016, 54, 473–479. [Google Scholar] [CrossRef]
- Bibaut-Renauld, C.; Burget, D.; Fouassier, J.-P.; Varelas, C.G.; Thomatos, J.; Tsagaropoulos, G.; Ryrfors, L.O.; Karlsson, O.J. Use of α-diketones as visible photoinitiators for the photocrosslinking of waterborne latex paints. J. Polym. Sci. A Polym. Chem. 2002, 40, 3171–3181. [Google Scholar] [CrossRef]
- Tomal, W.; Ortyl, J. Water-Soluble Photoinitiators in Biomedical Applications. Polymers 2020, 12, 1073. [Google Scholar] [CrossRef] [PubMed]
- Fairbanks, B.D.; Singh, S.P.; Bowman, C.N.; Anseth, K.S. Photodegradable, Photoadaptable Hydrogels via Radical-Mediated Disulfide Fragmentation Reaction. Macromolecules 2011, 44, 2444–2450. [Google Scholar] [CrossRef]
- Li, J.F.; Zhang, X.; Nie, J.; Zhu, X.Q. Visible light and water-soluble photoinitiating system based on the charge transfer complex for free radical photopolymerization. J. Photochem. Photobiol. A 2020, 402, 112803. [Google Scholar] [CrossRef]
- Wang, D.X.; Kaya, K.; Garra, P.; Fouassier, J.-P.; Graff, B.; Yagci, Y.; Lalevée, J. Sulfonium salt-based charge transfer complexes as dual thermal and photochemical polymerization initiators for composites and 3D printing. Polym. Chem. 2019, 10, 4690–4698. [Google Scholar] [CrossRef]
- Tasdelen, M.A.; Lalevée, J.; Yagci, Y. Photoinduced free radical promoted cationic polymerization 40 years after its discovery. Polym. Chem. 2020, 11, 1111–1121. [Google Scholar] [CrossRef]
- Baralle, A.; Garra, P.; Graff, B.; Morlet-Savary, F.; Dietlin, C.; Fouassier, J.P.; Lakhdar, S.; Lalevée, J. Iodinated Polystyrene for Polymeric Charge Transfer Complexes: Toward High-Performance Near-UV and Visible Light Macrophotoinitiators. Macromolecules 2019, 52, 3448–3453. [Google Scholar] [CrossRef]
- Garra, P.; Caron, A.; Mousawi, A.A.; Graff, B.; Morlet-Savary, F.; Dietlin, C.; Yagci, Y.; Fouassier, J.P.; Lalevée, J. Photochemical, Thermal Free Radical, and Cationic Polymerizations Promoted by Charge Transfer Complexes: Simple Strategy for the Fabrication of Thick Composites. Macromolecules 2018, 51, 7872–7880. [Google Scholar] [CrossRef]
- Telitel, S.; Dumur, F.; Campolo, D.; Poly, J.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Iron complexes as potential photocatalysts for controlled radical photopolymerizations: A tool for modifications and patterning of surfaces. J. Polym. Sci. A Polym. Chem. 2016, 54, 702–713. [Google Scholar] [CrossRef]
- Xu, Y.; Feng, T.; Yang, T.; Wei, H.; Yang, H.; Li, G.; Zhao, M.; Liu, S.; Huang, W.; Zhao, Q. Utilizing Intramolecular Photoinduced Electron Transfer to Enhance Photothermal Tumor Treatment of Aza-BODIPY-Based Near-Infrared Nanoparticles. ACS Appl. Mater. Interf. 2018, 10, 16299–16307. [Google Scholar] [CrossRef]
- Sun, K.; Xu, Y.Y.; Dumur, F.; Morlet-Savary, F.; Chen, H.; Dietlin, C.; Graff, B.; Lalevée, J.; Xiao, P. In silico rational design by molecular modeling of new ketones as photoinitiators in three-component photoinitiating systems: Application in 3D printing. Polym. Chem. 2020, 11, 2230–2242. [Google Scholar] [CrossRef]
- Minitti, M.P.; Weber, P.M. Time-Resolved Conformational Dynamics in Hydrocarbon Chains. Phys. Rev. Lett. 2007, 98, 253004. [Google Scholar] [CrossRef]
- Lamont, A.C.; Restaino, M.A.; Kima, M.J.; Sochol, R.D. A facile multi-material direct laser writing strategy. Lab Chip 2019, 19, 2340–2345. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Noirbent, G.; Zhang, Y.J.; Brunel, D.; Gigmes, D.; Morlet-Savary, F.; Graff, B.; Xiao, P.; Dumur, F. Lalevée, Novel D–π-A and A–π-D–π-A three-component photoinitiating systems based on carbazole/triphenylamino based chalcones and application in 3D and 4D printing. J. Polym. Chem. 2020, 11, 6512–6528. [Google Scholar] [CrossRef]
- Chen, H.; Noirbent, G.; Liu, S.H.; Brunel, D.; Graff, B.; Gigmes, D.; Zhang, Y.J.; Sun, K.; Morlet-Savary, F.; Xiao, P.; et al. Bis-chalcone derivatives derived from natural products as near-UV/visible light sensitive photoinitiators for 3D/4D printing. Mater. Chem. Front. 2021, 5, 901–916. [Google Scholar] [CrossRef]
- Tehfe, M.A.; Dumur, F.; Xiao, P.; Delgove, M.; Graff, B.; Fouassier, J.P.; Gigmes, D.; Lalevée, J. Chalcone derivatives as highly versatile photoinitiators for radical, cationic, thiol–ene and IPN polymerization reactions upon exposure to visible light. Polym. Chem. 2014, 5, 382–390. [Google Scholar] [CrossRef]
- Giacoletto, N.; Dumur, F. Recent Advances in bis-Chalcone-Based Photoinitiators of Polymerization: From Mechanistic Investigations to Applications. Molecules 2021, 26, 3192. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim-Ouali, M.; Dumur, F. Recent advances on chalcone-based photoinitiators of polymerization. Eur. Polym. J. 2021, 158, 110688. [Google Scholar] [CrossRef]
- Li, P.; Ge, P.; Ping, S.W.; Lin, W.T.; Zhang, X.H.; Wei, C.H.; Ren, Y. Photodegradation mechanism and influencing factors of asthma drug salmeterol under UV irradiation. J. Photochem. Photobiol. A 2021, 404, 112944. [Google Scholar] [CrossRef]









| Final Acrylate Function Conversions of PEG-DA Aqueous Monomers | |
|---|---|
| 0.1% dye | 8% |
| 0.5% dye | 33% |
| 0.1% [dye-TEA] | 8% |
| 0.5% [dye-TEA] | 75% |
| 1% [dye-TEA] | 84% |
| 2% [dye-TEA] | 86% |
| 3% [dye-TEA] | 82% |
| 4% [dye-TEA] | 72% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Vahdati, M.; Xiao, P.; Dumur, F.; Lalevée, J. Water-Soluble Visible Light Sensitive Photoinitiating System Based on Charge Transfer Complexes for the 3D Printing of Hydrogels. Polymers 2021, 13, 3195. https://doi.org/10.3390/polym13183195
Chen H, Vahdati M, Xiao P, Dumur F, Lalevée J. Water-Soluble Visible Light Sensitive Photoinitiating System Based on Charge Transfer Complexes for the 3D Printing of Hydrogels. Polymers. 2021; 13(18):3195. https://doi.org/10.3390/polym13183195
Chicago/Turabian StyleChen, Hong, Mehdi Vahdati, Pu Xiao, Frédéric Dumur, and Jacques Lalevée. 2021. "Water-Soluble Visible Light Sensitive Photoinitiating System Based on Charge Transfer Complexes for the 3D Printing of Hydrogels" Polymers 13, no. 18: 3195. https://doi.org/10.3390/polym13183195