Development, Characterization and Valuable Use of Novel Dosimeter Film Based on PVA Polymer Doped Nitro Blue Tetrazolium Dye and AgNO3 for the Accurate Detection of Low X-ray Doses
Abstract
:1. Introduction
2. Experiment
2.1. Materials
2.2. Characterization of Film Nanocomposites
2.3. Preparation of PVA/NBT Nanocomposite Films
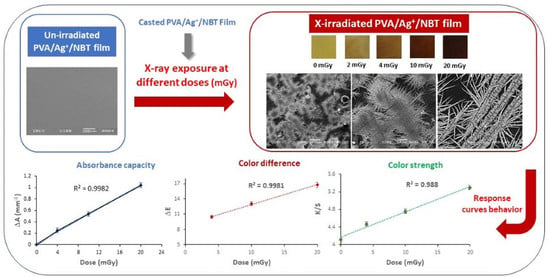
3. Results and Discussion
3.1. Post-Irradiation Stability
3.2. Direct Perception of Color Change after Irradiation
3.3. UV/Visible Absorption Spectra
3.4. Response Curve
3.5. X-ray Diffraction Study
3.6. Mechanical Characterization
3.7. SEM Morphological Analysis
3.8. Colorimetric Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Mohammadi, M. Book review: Advanced Radiation Protection Dosimetry edited by Shaheen A. Dewji and Nolan E. Hertel. Phys. Eng. Sci. Med. 2020, 43, 727–728. [Google Scholar] [CrossRef]
- Alevra, A.V.; Klein, H.; Knauf, K.; Wittstock, J. Neutron field spectrometry for radiation protection dosimetry purposes. Radiat. Prot. Dosim. 1992, 44, 223–226. [Google Scholar] [CrossRef]
- Alterio, D.; D’Ippolito, E.; Vischioni, B.; Fossati, P.; Gandini, S.; Bonora, M.; Ronchi, S.; Vitolo, V.; Mastella, E.; Magro, G.; et al. Mixed-Beam Approach in Locally Advanced Nasopharyngeal Carcinoma: IMRT Followed by Proton Therapy Boost Versus IMRT-Only. Evaluation of Toxicity and Efficacy. Acta Oncol. 2020, 59, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Guardiola, C.; Fleta, C.; Quirion, D.; Pellegrini, G.; Gómez, F. Silicon 3D Microdetectors for Microdosimetry in Hadron Therapy. Micromachines 2020, 11, 1053. [Google Scholar] [CrossRef]
- Bhatt, B.C.; Kulkarni, M.S. Worldwide Status of Personnel Monitoring using Thermoluminescent (TL), Optically Stimulated Luminescent (OSL) and Radiophotoluminescent (RPL) Dosimeters. Int. J. Lumin. Appl. 2013, 3, 6–10. [Google Scholar]
- Pradhan, A.S.; Lee, J.I.; Kim, J.L. On the scenario of passive dosimeters in personnel monitoring: Relevance to diagnostic radiology and fluoroscopy-based interventional cardiology. J. Med. Phys. 2016, 41, 81–84. [Google Scholar] [CrossRef]
- Wesolowska, P.E.; Cole, A.; Santos, T.; Bokulic, T.; Kazantsev, P.; Izewska, J. Characterization of three solid state dosimetry systems for use in high energy photon dosimetry audits in radiotherapy. Radiat. Meas. 2017, 106, 556–562. [Google Scholar] [CrossRef]
- Pati, S.; Chatterji, A.; Dash, B.P.; Raveen Nelson, B.; Sarkar, T.; Shahimi, S.; Atan Edinur, H.; Binti Abd Manan, T.S.; Jena, P.; Mohanta, Y.K.; et al. Structural Characterization and Antioxidant Potential of Chitosan by γ-Irradiation from the Carapace of Horseshoe Crab. Polymers 2020, 12, 2361. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhang, M.; He, Y.; Zhang, M.; Shen, R.; Zhang, Y.; Wang, M.; Wu, G. Fabrication and Potential Applications of Highly Durable Superhydrophobic Polyethylene Terephthalate Fabrics Produced by In-Situ Zinc Oxide (ZnO) Nanowires Deposition and Polydimethylsiloxane (PDMS) Packaging. Polymers 2020, 12, 2333. [Google Scholar] [CrossRef]
- Tapia-Guerrero, Y.S.; Del Prado-Audelo, M.L.; Borbolla-Jiménez, F.V.; Giraldo Gomez, D.M.; García-Aguirre, I.; Colín-Castro, C.A.; Morales-González, J.A.; Leyva-Gómez, G.; Magaña, J.J. Effect of UV and Gamma Irradiation Sterilization Processes in the Properties of Different Polymeric Nanoparticles for Biomedical Applications. Materials 2020, 13, 1090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, Y.K.; Lui, C.N.P.; Chen, Y.W.; Chou, S.W.; Raine, E.; Chou, P.T.; Yung, K.K.L.; Tsang, S.C.E. Engineering of Single Magnetic Particle Carrier for Living Brain Cell Imaging: A Tunable T1-/T2-/Dual-Modal Contrast Agent for Magnetic Resonance Imaging Application. Chem. Mater. 2017, 29, 4411–4417. [Google Scholar] [CrossRef]
- Li, C.H.; Kuo, T.R.; Su, H.J.; Lai, W.Y.; Yang, P.C.; Chen, J.S.; Wang, D.Y.; Wu, Y.C.; Chen, C.C. Fluorescence-Guided Probes of Aptamer-Targeted Gold Nanoparticles with Computed Tomography Imaging Accesses for in Vivo Tumor Resection. Sci. Rep. 2015, 5, 15675. [Google Scholar] [CrossRef]
- Inaba, Y.; Nakamura, M.; Zuguchi, M.; Chida, K. Development of Novel Real-Time Radiation Systems Using 4-Channel Sensors. Sensors 2020, 20, 2741. [Google Scholar] [CrossRef]
- Phong Vo, P.; Ngoc Doan, H.; Kinashi, K.; Sakai, W.; Tsutsumi, N.; Phu Huynh, D. X-ray Visualization and Quantification Using Fibrous Color Dosimeter Based on Leuco Dye. Appl. Sci. 2020, 10, 3798. [Google Scholar] [CrossRef]
- Diffey, B. The Early Days of Personal Solar Ultraviolet Dosimetry. Atmosphere 2020, 11, 125. [Google Scholar] [CrossRef] [Green Version]
- Kržanović, N.; Stanković, K.; Živanović, M.; Đaletić, M.; Ciraj-Bjelac, O. Development and testing of a low cost radiation protection instrument based on an energy compensated Geiger-Müller tube. Radiat. Phys. Chem. 2019, 164, 108358. [Google Scholar] [CrossRef]
- Glais, E.; Massuyeau, F.; Gautier, R. Tuning the oxidation states of dopants: A strategy for the modulation of material photoluminescence properties. Chem. A Eur. J. 2020, 27, 905–914. [Google Scholar] [CrossRef]
- William, J.E. Tutorial on the Role of Cyclopentadienyl Ligands in the Discovery of Molecular Complexes of the Rare-Earth and Actinide Metals in New Oxidation States. Organometallics 2016, 35, 3088–3100. [Google Scholar]
- Bhaskar, S.; Goswami, M.; Shobha, S.; Prakasan, V.; Krishnan, M.; Ghosh, S.K. Thermoluminescence and electron paramagnetic resonance study on rare earth/transition metal doped lithium borate glasses for dosimetry applications. J. Lumin. 2019, 216, 116725. [Google Scholar]
- Chandler, J.R.; Sholom, S.; McKeever, S.W.S.; Hall, H.L. Thermoluminescence and phototransferred thermoluminescence dosimetry on mobile phone protective touchscreen glass. J. Appl. Phys. 2019, 126, 074901. [Google Scholar] [CrossRef]
- Vo, P.P.; Doan, H.N.; Kinashi, K.; Sakai, W.; Tsutsumi, N. X-ray composite fibrous color dosimeter based on 10,12-pentacosadiynoic acid. Dyes Pigments 2021, 191, 109356. [Google Scholar] [CrossRef]
- AL Zahrany, A.; Rabaeh, K.; Eyadeh, M.; Basfar, A. Dosimetric evaluation of methyl red radiochromic film for radiation processing. Pigment. Resin Technol. 2020, 50, 157–162. [Google Scholar] [CrossRef]
- Ozkan Loch, C.; Eichenberger, M.A.; Togno, M.; Zinsli, S.P.; Egloff, M.; Papa, A.; Ischebeck, R.; Lomax, A.J.; Peier, P.; Safai, S. Characterization of a Low-Cost Plastic Fiber Array Detector for Proton Beam Dosimetry. Sensors 2020, 20, 5727. [Google Scholar] [CrossRef]
- Santos, T.; Ventura, T.; Lopes, M.d.C. A review on radiochromic film dosimetry for dose verification in high energy photon beams. Radiat. Phys. Chem. 2020, 179, 109217. [Google Scholar]
- Sąsiadek, E.; Jaszczak, M.; Skwarek, J.; Kozicki, M. NBT-Pluronic F-127 Hydrogels Printed on Flat Textiles as UV Radiation Sensors. Materials 2021, 14, 3435. [Google Scholar] [CrossRef] [PubMed]
- Seito, H.; Ichikawa, T.; Hanaya, H.; Sato, Y.; Kaneko, H.; Haruyama, Y.; Watanabe, H.; Kojima, T. Application of clear polymethylmethacrylate dosimeter Radix W to a few MeV electron in radiation processing. Radiat. Phys. Chem. 2009, 78, 961–965. [Google Scholar] [CrossRef]
- Rabaeh, K.A.; Basfar, A.A. A polystyrene film dosimeter containing dithizone dye for high dose applications of gamma-ray source. Radiat. Phys. Chem. 2020, 170, 108646. [Google Scholar] [CrossRef]
- Alminderej, F.M.; Ammar, C.; El-Ghoul, Y. Functionalization, characterization and microbiological performance of new biocompatible cellulosic dressing grafted chitosan and Suaeda fruticosa polysaccharide extract. Cellulose 2021. [Google Scholar] [CrossRef]
- EL-Ghoul, Y.; Ammar, C.; Alminderej, F.M.; Shafiquzzaman, M. Design and Evaluation of a New Natural Multi-Layered Biopolymeric Adsorbent System-Based Chitosan/Cellulosic Nonwoven Material for the Biosorption of Industrial Textile Effluents. Polymers 2021, 13, 322. [Google Scholar] [CrossRef]
- Ammar, C.; Alminderej, F.M.; EL-Ghoul, Y.; Jabli, M.; Shafiquzzaman, M. Preparation and Characterization of a New Polymeric Multi-Layered Material Based K-Carrageenan and Alginate for Efficient Bio-Sorption of Methylene Blue Dye. Polymers 2021, 13, 411. [Google Scholar] [CrossRef]
- El-Ghoul, Y. Biological and microbiological performance of new polymer-based chitosan and synthesized aminocyclodextrin finished polypropylene abdominal wall prosthesis biomaterial. Text. Res. J. 2020, 90, 2690–2702. [Google Scholar] [CrossRef]
- Alminderej, M.F.; El-Ghoul, Y. Synthesis and study of a new biopolymer-based chitosan/hematoxylin grafted to cotton wound dressings. J. Appl. Polym. Sci. 2019, 136, 47625. [Google Scholar] [CrossRef]
- Dhibar, S.; Sahoo, S.; Das, C.K. Fabrication of transition-metal-doped polypyrrole/multiwalled carbon nanotubes nanocomposites for supercapacitor applications. J. Appl. Polym. Sci. 2013, 130, 554–556. [Google Scholar] [CrossRef]
- Carraro, M.; Gross, S. Hybrid Materials Based on the Embedding of Organically Modified Transition Metal Oxoclusters or Polyoxometalates into Polymers for Functional Applications: A Review. Materials 2014, 7, 3956–3989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ueda, K.; Kakuta, T.; Tanaka, K.; Chujo, Y. High Refractive-Index Hybrids Consisting of Water-Soluble Matrices with Bipyridine-Modified Polyhedral Oligomeric Silsesquioxane and Lanthanoid Cations. Polymers 2020, 12, 1560. [Google Scholar] [CrossRef] [PubMed]
- Khanna, P.K.; Singh, N.; Charan, S.; Viswanath, K.A. Synthesis of Ag/polyaniline nano composite via an in situ photo-redox mechanism. Mater. Chem. Phys. 2005, 92, 214–219. [Google Scholar] [CrossRef]
- Monti, O.L.A.; Fourkas, J.T.; Nesbitt, D.J. DiffractionLimited Photo generation and Characterization of Silver Nanoparticles. J. Phys. Chem. B 2004, 108, 1604–1612. [Google Scholar] [CrossRef]
- Osman, M.I.; Abdalla, D.M.; Elfaki, H.A.; Yagoub, Y.A.K.; Ibrahim Hussein, K.; Eldoma, M.A.; Omer Al-atta, N.; Mohamed Osman Siddig, Y. Effects of additive and gamma irradiation on the structural and optical properties of polyvinyl alcohol doped with silver nitrate. Glob. Sci. J. 2021, 9, 1780–1792. [Google Scholar]
- Raouafi, A.; Daoudi, M.; Jouini, K.; Charradi, K.; Hamzaoui, A.H.; Blaise, P.; Farah, K.; Hosni, F. Effect of gamma irradiation on the color, structure and morphology of nickel-doped polyvinyl alcohol films: Alternative use as dosimeter or irradiation indicator. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2018, 425, 4–10. [Google Scholar] [CrossRef]
- Arshak, K.; Korostynska, O.; Harris, J.; Morris, D.; Arshak, A.; Jafer, E. Properties of BGO Thin Films under the Influence of Gamma Radiation. Thin Solid Film. 2008, 516, 1493–1498. [Google Scholar] [CrossRef]
- Arshak, A.; Zleenti, S.; Arshak, K. γ-Radiation Sensor Using Optical and Electrical Properties of Manganese Phthalocyanine (MnPc) Thick Film. Sonsors 2002, 2, 174–184. [Google Scholar] [CrossRef] [Green Version]
- Eid, S.; Ebraheem, S.; Abdel-Kader, N. Study the Effect of Gamma Radiation on the Optical Energy Gap of Poly (Vinyl Alcohol) Based Ferrotitanium Alloy Film: Its Possible Use in Radiation Dosimetry. Open J. Polym. Chem. 2014, 4, 21–30. [Google Scholar] [CrossRef] [Green Version]
- Doyan, A.; Susilawati, S.; Prayogi, S.; Bilad, M.R.; Arif, M.F.; Ismail, N.M. Polymer Film Blend of Polyvinyl Alcohol, Trichloroethylene and Cresol Red for Gamma Radiation Dosimetry. Polymers 2021, 13, 1866. [Google Scholar] [CrossRef] [PubMed]
- Susilawati, S.; Prayogi, S.; Arif, M.F.; Ismail, N.M.; Bilad, M.R.; Asy’ari, M. Optical Properties and Conductivity of PVA–H3PO4 (Polyvinyl Alcohol–Phosphoric Acid) Film Blend Irradiated by γ-Rays. Polymers 2021, 13, 1065. [Google Scholar] [CrossRef] [PubMed]
- Alashrah, S.; El-Ghoul, Y.; Omer, M.A.A. Synthesis and Characterization of a New Nanocomposite Film Based on Polyvinyl Alcohol Polymer and Nitro Blue Tetrazolium Dye as a Low Radiation Dosimeter in Medical Diagnostics Application. Polymers 2021, 13, 1815. [Google Scholar] [CrossRef] [PubMed]
- Eldoma, A.M.; Al-atta, N.O.; Ahmed, K.Y. Effect of Doping Concentrations on the Sensitivity of PVA/AgNO3 Films to low X-ray doses. Int. J. Adv. Sci. Eng. Technol. 2019, 6, 8460–8466. [Google Scholar]
- Lee, H.C. Introduction to Color Imaging Science; Cambridge University Press: New York, NY, USA, 2005. [Google Scholar]
- Kuehni, R.G. Color: An Introduction to Practice and Principles, 2nd ed.; John Wiley: Hoboken, NJ, USA, 2005. [Google Scholar]
- Schanda, J. Colorimetry: Understanding the CIE System; John Wiley: Hoboken, NJ, USA, 2007. [Google Scholar]
- Shams-Nateri, A. Effect of a standard colorimetric observer on the reconstruction of reflectance spectra of coloured fabrics. Coloration Technol. 2008, 124, 14–18. [Google Scholar] [CrossRef]
- McGrath, J.R.; Beck, M.; Hill, M.E. Replicating Red: Analysis of ceramic slip color with CIELAB color data. J. Archaeol. Sci. Rep. 2017, 14, 432–438. [Google Scholar] [CrossRef]
- Johnston, W.M.; Kao, E.C. Assessment of Appearance Match by Visual Observation and Clinical Colorimetry. J. Dent. Res. 1989, 68, 819–822. [Google Scholar] [CrossRef]
- El Gohary, M.I.; Soliman, Y.S.; Amin, E.A.; Gawad, M.H.A.; Desouky, O.S. Effect of perchloric acid on the performance of the Fricke xylenol gel dosimeter. Appl. Radiat. Isot. 2016, 113, 66–69. [Google Scholar] [CrossRef]
- Abdel-Fattah, A.A.; Beshir, W.B.; Hassan, H.M.; Soliman, Y.S. Radiation-induced coloration of nitro blue tetrazolium gel dosimeter for low dose applications. Radiat. Meas. 2017, 100, 18–26. [Google Scholar] [CrossRef]
- Emi-Reynolds, G.; Kovacs, A.; Fletcher, J.J. Dosimetry characterization of tetrazolium violet-polyvinylalcohol films. Radiat. Phys. Chem. 2007, 76, 1519–1522. [Google Scholar] [CrossRef]
- Pikaev, A.K.; Kriminskaya, Z.K. Use of tetrazolium salts in dosimetry of ionizing radiation. Radiat. Phys. Chem. 1998, 52, 555–561. [Google Scholar] [CrossRef]
- Kovacs, A.; Baranyai, M.; Wojn!arovits, L.; Moussa, A.; Othman, I.; McLaughlin, W.L. Aqueous-ethanol nitro blue tetrazolium solutions for high dose dosimetry. Radiat. Phys.Chem. 1999, 55, 799–803. [Google Scholar] [CrossRef]
- Moussa, A.; Baranyai, M.; Wojnárovits, L.; Kovács, A.; McLaughlin, W. Dosimetry characteristics of the nitro blue tetrazolium-polyvinylalcohol film for high dose applications. Radiat. Phys. Chem. 2003, 68, 1011–1015. [Google Scholar] [CrossRef]
- Khanna, P.K.; Singh, N.; Charan, S.; Subbarao, V.V.V.S.; Gokhale, R.; Mulik, U.P. Synthesis and characterization of Ag/PVA nanocomposite by chemical reduction method. Mater. Chem. Phys. 2005, 93, 117–121. [Google Scholar] [CrossRef]
- Ramnani, S.P.; Biswal, J.; Sabharwal, S. Synthesis of silver nanoparticles supported on silica aerogel using gamma radiolysis. Radiat. Phys. Chem. 2007, 76, 1290–1294. [Google Scholar] [CrossRef]
- Agabekov, V.; Ivanova, N.; Dlugunovich, V.; Vostchula, I. Optical Properties of Polyvinyl Alcohol FilmsModified with Silver Nanoparticles. J. Nanomater. 2012, 2012, 10. [Google Scholar] [CrossRef]
- Eisa, H.W.; Abdel-Moneam, Y.K.; Shaaban, Y.; Abdel-Fattah, A.A.; Zeid, A.M.A. Gamma-irradiation assisted seeded growth of Ag nanoparticles within PVA matrix. Mater. Chem. Phys. 2011, 128, 109–113. [Google Scholar] [CrossRef]
- Bhat, N.V.; Nate, M.M.; Kurup, M.B.; Bambole, V.A.; Sabharwal, S. Effect of γ-radiation on the structure and morphology of polyvinyl alcohol films. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2005, 237, 585–592. [Google Scholar] [CrossRef]
- Akhavan, A.; Khoylou, F.; Ataeivarjovi, E. Preparation and characterization of gamma irradiated Starch/PVA/ZnO nanocomposite films. Radiat. Phys. Chem. 2017, 138, 49–53. [Google Scholar] [CrossRef]
- Chen, S.A.; Fang, W.G. Electrically conductive polyaniline-poly (vinyl alcohol) composite films: Physical properties and morphological structures. Macromolecules 1991, 24, 1242–1248. [Google Scholar] [CrossRef]
- Liu, J.; Lin, Q.; Zhou, Y.; Dai, J.; Han, Y. Shape Controllable Synthesis of Silver Particles by Selecting the Crystallization Routes. KONA Powder Part. J. 2020, 37, 166–175. [Google Scholar] [CrossRef] [Green Version]








| Radiation Dose | L* | a* | b* | ΔE | K/S |
|---|---|---|---|---|---|
| 0 | 51.61 | 2.04 | 12.14 | - | 4.11 |
| 4 | 52.80 | 2.28 | 13.44 | 10.47 | 4.47 |
| 10 | 54.90 | 2.81 | 15.27 | 13.05 | 4.76 |
| 20 | 57.73 | 3.65 | 17.83 | 16.73 | 5.30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alashrah, S.; El-Ghoul, Y.; Almutairi, F.M.; Omer, M.A.A. Development, Characterization and Valuable Use of Novel Dosimeter Film Based on PVA Polymer Doped Nitro Blue Tetrazolium Dye and AgNO3 for the Accurate Detection of Low X-ray Doses. Polymers 2021, 13, 3140. https://doi.org/10.3390/polym13183140
Alashrah S, El-Ghoul Y, Almutairi FM, Omer MAA. Development, Characterization and Valuable Use of Novel Dosimeter Film Based on PVA Polymer Doped Nitro Blue Tetrazolium Dye and AgNO3 for the Accurate Detection of Low X-ray Doses. Polymers. 2021; 13(18):3140. https://doi.org/10.3390/polym13183140
Chicago/Turabian StyleAlashrah, Saleh, Yassine El-Ghoul, Faisal Muteb Almutairi, and Mohammed Ahmed Ali Omer. 2021. "Development, Characterization and Valuable Use of Novel Dosimeter Film Based on PVA Polymer Doped Nitro Blue Tetrazolium Dye and AgNO3 for the Accurate Detection of Low X-ray Doses" Polymers 13, no. 18: 3140. https://doi.org/10.3390/polym13183140