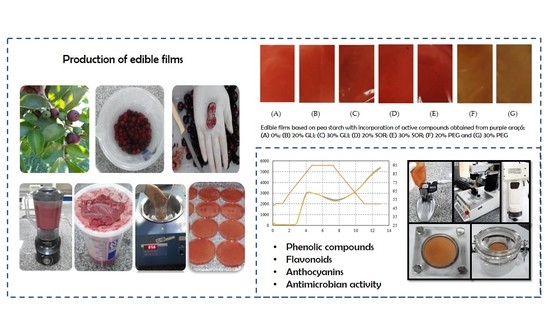
Production of Edible Films Based on Pea Starch with Incorporation of Active Compounds Obtained from the Purple Araçá (Psidium myrtoides)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Pea Starch Characterization
2.2.1. Starch Granule Microstructure
2.2.2. Amylose Content
2.2.3. Paste Properties (RVA)
2.3. Production of Pea Starch Films
2.4. Characterization of Edible Films
2.4.1. Visual Appearance and Thickness
2.4.2. Color Parameters
2.4.3. Contact Angle
2.4.4. Water Vapor Permeability (WVP)
2.4.5. Solubility in Water
2.4.6. Mechanical Properties
2.5. Content of Total Phenolic Compounds and Antioxidant Activity
2.5.1. Phenolic Compounds
2.5.2. Flavonoids
2.5.3. Anthocyanins
2.6. Antimicrobial Activity
2.7. Statistical Analysis
3. Results and Discussion
3.1. Pea Starch Microstructure, Amylose Content, and Paste Properties
3.2. Film Characterization
3.2.1. Visual Appearance and Color Parameters
3.2.2. Contact Angle, Water Vapor Permeability and Water Solubility
3.2.3. Mechanical Properties
3.3. Total Phenolic Compounds Content and Antioxidant Activity
3.4. Antimicrobial Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Genskowsky, E.; Puente, L.A.; Pérez-Álvarez, J.A.; Fernandez-Lopez, J.; Muñoz, L.A.; Viuda-Martos, M. Assessment of antibacterial and antioxidant properties of chitosan edible films incorporated with maqui berry (Aristotelia chilensis). LWT Food Sci. Technol. 2015, 64, 1057–1062. [Google Scholar] [CrossRef]
- Otoni, C.G.; Avena-Bustillos, R.J.; Azeredo, H.M.C.; Lorevice, M.V.; Moura, M.R.; Mattoso, L.H.C.; McHugh, T.H. Recent Advances on Edible Films Based on Fruits and Vegetables—A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1151–1169. [Google Scholar] [CrossRef] [Green Version]
- Fonseca, L.M.; Henkes, A.K.; Bruni, G.P.; Viana, L.A.N.; de Moura, C.M.; Flores, W.H.; Galio, A.F. Fabrication and Characterization of Native and Oxidized Potato Starch Biodegradable Films. Food Biophys. 2018, 13, 163–174. [Google Scholar] [CrossRef]
- González, A.; Gastelú, G.; Barrera, G.N.; Ribotta, P.D.; Álvarez Igarzabal, C.I. Preparation and characterization of soy protein films reinforced with cellulose nanofibers obtained from soybean by-products. Food Hydrocoll. 2019, 89, 758–764. [Google Scholar] [CrossRef]
- Hu, Y.; Shi, L.; Ren, Z.; Hao, G.; Chen, J.; Weng, W. Characterization of emulsion films prepared from soy protein isolate at different preheating temperatures. J. Food Eng. 2021, 309, 110697. [Google Scholar] [CrossRef]
- Zhou, D.; Ma, Z.; Yin, X.; Hu, X.; Boye, J.I. Structural characteristics and physicochemical properties of field pea starch modified by physical, enzymatic, and acid treatments. Food Hydrocoll. 2019, 93, 386–394. [Google Scholar] [CrossRef]
- Biduski, B.; da Silva, W.M.F.; Colussi, R.; El Halal, S.L.D.M.; Lim, L.T.; Dias, Á.R.G.; da Rosa Zavareze, E. Starch hydrogels: The influence of the amylose content and gelatinization method. Int. J. Biol. Macromol. 2018, 113, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Laohakunjit, N.; Noomhorm, A. Effect of plasticizers on mechanical and barrier properties of rice starch film. Starch/Staerke 2004, 56, 348–356. [Google Scholar] [CrossRef]
- Otoni, C.G.; de Moura, M.R.; Aouada, F.A.; Camilloto, G.P.; Cruz, R.S.; Lorevice, M.V.; Soares, N.d.F.F.; Mattoso, L.H.C. Antimicrobial and physical-mechanical properties of pectin/papaya puree/cinnamaldehyde nanoemulsion edible composite films. Food Hydrocoll. 2014, 41, 188–194. [Google Scholar] [CrossRef]
- Azeredo, H.M.C.; Morrugares-Carmona, R.; Wellner, N.; Cross, K.; Bajka, B.; Waldron, K.W. Development of pectin films with pomegranate juice and citric acid. Food Chem. 2016, 198, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, G.d.M.; Filgueiras, C.T.; Garcia, V.A.D.S.; de Carvalho, R.A.; Velasco, J.I.; Fakhouri, F.M. Antimicrobial activity and gc-ms profile of copaiba oil for incorporation into xanthosoma mafaffa schott starch-based films. Polymers 2020, 12, 2883. [Google Scholar] [CrossRef] [PubMed]
- Fakhouri, F.M.; Nogueira, G.F.; de Oliveira, R.A.; Velasco, J.I. Bioactive edible films based on arrowroot starch incorporated with cranberry powder: Microstructure, thermal properties, ascorbic acid content and sensory analysis. Polymers 2019, 11, 1650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nogueira, G.F.; Fakhouri, F.M.; Velasco, J.I.; de Oliveira, R.A. Active edible films based on arrowroot starch with microparticles of blackberry pulp obtained by freeze-drying for food packaging. Polymers 2019, 11, 1382. [Google Scholar] [CrossRef] [Green Version]
- Malherbi, N.M.; Schmitz, A.C.; Grando, R.C.; Bilck, A.P.; Yamashita, F.; Tormen, L.; Fakhouri, F.M.; Velasco, J.I.; Bertan, L.C. Corn starch and gelatin-based films added with guabiroba pulp for application in food packaging. Food Packag. Shelf Life 2019, 19, 140–146. [Google Scholar] [CrossRef]
- Farias, M.G.; Fakhouri, F.M.; Piler de Carvalho, C.W.; Ascheri, J.L.R. Caracterização físico-química de filmes comestíveis de amido adicionado de acerola (Malphigia emarginata D.C.). Quim. Nova 2012, 35, 546–552. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Liu, W.; Tian, B.; Li, D.; Liu, C.; Jiang, B.; Feng, Z. Preparation and characterization of coating based on protein nanofibers and polyphenol and application for salted duck egg yolks. Foods 2020, 9, 449. [Google Scholar] [CrossRef] [Green Version]
- Jiang, B.; Wang, L.; Zhu, M.; Wu, S.; Wang, X.; Li, D.; Liu, C.; Feng, Z.; Tian, B. Separation, structural characteristics and biological activity of lactic acid bacteria exopolysaccharides separated by aqueous two-phase system. LWT 2021, 147, 111617. [Google Scholar] [CrossRef]
- Lorenzi, H. Árvores Brasileiras: Manual de Identificação e Cultivo de Plantas Arbóreas Nativas do Brasil, 2nd ed.; Plantarum: São Paulo, Brazil, 1998. [Google Scholar]
- Sobral, M.; Proença, C.; Souza, M.; Mazine, F.; Lucas, E. Myrtaceae in Lista de Espécies da Flora do Brasil. Available online: http://floradobrasil.jbrj.gov.br/jabot/floradobrasil/FB10874 (accessed on 25 February 2019).
- Balisteiro, D.M.; Alezandro, M.R.; Genovese, M.I. Characterization and effect of clarified araçá (Psidium guineenses Sw.) juice on postprandial glycemia in healthy subjects. Ciência Tecnol. Aliment. 2013, 33, 66–74. [Google Scholar] [CrossRef] [Green Version]
- Denardin, C.C.; Hirsch, G.E.; Da Rocha, R.F.; Vizzotto, M.; Henriques, A.T.; Moreira, J.C.F.; Guma, F.T.C.R.; Emanuelli, T. Antioxidant capacity and bioactive compounds of four Brazilian native fruits. J. Food Drug Anal. 2015, 23, 387–398. [Google Scholar] [CrossRef] [Green Version]
- Stafussa, A.P.; Maciel, G.M.; Rampazzo, V.; Bona, E.; Makara, C.N.; Junior, B.D.; Haminiuk, C.W.I. Bioactive compounds of 44 traditional and exotic Brazilian fruit pulps: Phenolic compounds and antioxidant activity. Int. J. Food Prop. 2018, 21, 106–118. [Google Scholar] [CrossRef]
- Freitas, T.S.M.; Rodrigues, G.M.; Fakhouri, F.M.; Silva, C.; Cardoso, C.A.L.; Velasco, J.I.; Filgueira, C.T.; Garcia, V.A.S. Application of the Box–Behnken experimental design for the extraction of phenolic compounds from araçá-roxo (Psidium myrtoides). J. Food Process. Preserv. 2021, 45, e15260. [Google Scholar] [CrossRef]
- Da Silva, L.R.; Piler de Carvalho, C.W.; Velasco, J.I.; Fakhouri, F.M. Extraction and characterization of starches from pigmented rice. Int. J. Biol. Macromol. 2020, 156, 485–493. [Google Scholar] [CrossRef]
- Nogueira, G.F.; Fakhouri, F.M.; de Oliveira, R.A. Effect of incorporation of blackberry particles on the physicochemical properties of edible films of arrowroot starch. Dry. Technol. 2018, 37, 448–457. [Google Scholar] [CrossRef]
- American Society for Testing and Materials (ASTM). Standard Test Methods for Water Vapor Transmission of Materials; Method E96 e 80; American Society for Testing and Materials (ASTM): West Conshohocken, PA, USA, 1989. [Google Scholar]
- Gontard, N.; Guilbert, S.; Cuq, J.-L. Edible Wheat Gluten Films: Influence of the Main Process Variables on Film Properties using Response Surface Methodology. J. Food Sci. 1992, 57, 190–195. [Google Scholar] [CrossRef]
- American Society for Testing and Materials (ASTM). Standard Test Methods for Tensile Properties of Thim Plastic Sheeting; Method D 882-83; American Society for Testing and Materials (ASTM): West Conshohocken, PA, USA, 1980. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Lin, J.Y.; Tang, C.Y. Determination of total phenolic and flavonoid contents in selected fruits and vegetables, as well as their stimulatory effects on mouse splenocyte proliferation. Food Chem. 2007, 101, 140–147. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.W.; Wroslstad, R.E. Determination of total monomeric anthocyan in pigment contente of fruit juices, beverages, natural colorants, andwines by the pH differential method: Collaborative study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef] [Green Version]
- Nogueira, G.F.; Fakhouri, F.M.; de Oliveira, R.A. Extraction and characterization of arrowroot (Maranta arundinaceae L.) starch and its application in edible films. Carbohydr. Polym. 2018, 186, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.J.; Lin, S.L.; Wolfson, H.; Nussinov, R. Studies of protein-protein interfaces: A statistical analysis of the hydrophobic effect. Protein Sci. 1997, 6, 53–64. [Google Scholar] [CrossRef]
- Leonel, M.; Cereda, M.P. Caracterização Físico-Química De Algumas Tuberosas Amiláceas. Ciênc. Tecnol. Aliment. Campinas 2002, 22, 65–69. [Google Scholar] [CrossRef] [Green Version]
- Rosenthal, F.R.T.; Mello Alzira, P.; Pelegrino Sandra, L.; Nakamura, T. Amidos de mandioca. II. Estudos de estructura, em variedades de Minas Gerais. Rev. Bras. Tecnol. 1973, 4, 7–18. [Google Scholar]
- Saberi, B.; Chockchaisawasdee, S.; Golding, J.B.; Scarlett, C.J.; Stathopoulos, C.E. Physical and mechanical properties of a new edible film made of pea starch and guar gum as affected by glycols, sugars and polyols. Int. J. Biol. Macromol. 2017, 104, 345–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Razavi, S.M.A.; Mohammad Amini, A.; Zahedi, Y. Characterisation of a new biodegradable edible film based on sage seed gum: Influence of plasticiser type and concentration. Food Hydrocoll. 2015, 43, 290–298. [Google Scholar] [CrossRef]
- Vogler, E.A. Structure and reactivity of water at biomaterial surfaces. Adv. Colloid Interface Sci. 1998, 74, 69–117. [Google Scholar] [CrossRef]
- Pająk, P.; Przetaczek-Rożnowska, I.; Juszczak, L. Development and physicochemical, thermal and mechanical properties of edible films based on pumpkin, lentil and quinoa starches. Int. J. Biol. Macromol. 2019, 138, 441–449. [Google Scholar] [CrossRef]
- Feng, M.; Yu, L.; Zhu, P.; Zhou, X.; Liu, H.; Yang, Y.; Zhou, J.; Gao, C.; Bao, X.; Chen, P. Development and preparation of active starch films carrying tea polyphenol. Carbohydr. Polym. 2018, 196, 162–167. [Google Scholar] [CrossRef]
- Edhirej, A.; Sapuan, S.M.; Jawaid, M.; Zahari, N.I. Effect of various plasticizers and concentration on the physical, thermal, mechanical, and structural properties of cassava-starch-based films. Starch/Staerke 2017, 69, 1–11. [Google Scholar] [CrossRef]
- Rangel-Marrón, M.; Mani-López, E.; Palou, E.; López-Malo, A. Effects of alginate-glycerol-citric acid concentrations on selected physical, mechanical, and barrier properties of papaya puree-based edible films and coatings, as evaluated by response surface methodology. LWT Food Sci. Technol. 2019, 101, 83–91. [Google Scholar] [CrossRef]
- Reis, L.C.B.; De Souza, C.O.; Da Silva, J.B.A.; Martins, A.C.; Nunes, I.L.; Druzian, J.I. Active biocomposites of cassava starch: The effect of yerba mate extract and mango pulp as antioxidant additives on the properties and the stability of a packaged product. Food Bioprod. Process. 2015, 94, 382–391. [Google Scholar] [CrossRef]
- Nogueira, G.F.; Soares, C.T.; Cavasini, R.; Fakhouri, F.M.; de Oliveira, R.A. Bioactive films of arrowroot starch and blackberry pulp: Physical, mechanical and barrier properties and stability to pH and sterilization. Food Chem. 2019, 275, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Prietto, L.; Mirapalhete, T.C.; Pinto, V.Z.; Hoffmann, J.F.; Vanier, N.L.; Lim, L.T.; Guerra Dias, A.R.; da Rosa Zavareze, E. pH-sensitive films containing anthocyanins extracted from black bean seed coat and red cabbage. LWT Food Sci. Technol. 2017, 80, 492–500. [Google Scholar] [CrossRef]
- Silva-Pereira, M.C.; Teixeira, J.A.; Pereira-Júnior, V.A.; Stefani, R. Chitosan/corn starch blend films with extract from Brassica oleraceae (red cabbage) as a visual indicator of fish deterioration. LWT Food Sci. Technol. 2015, 61, 258–262. [Google Scholar] [CrossRef] [Green Version]
- Garcia, V.A.d.S.; Borges, J.G.; Osiro, D.; Vanin, F.M.; de Carvalho, R.A. Orally disintegrating films based on gelatin and pregelatinized starch: New carriers of active compounds from acerola. Food Hydrocoll. 2020, 101, 105518. [Google Scholar] [CrossRef]




| Formulations | Color Parameters | ||
|---|---|---|---|
| L* | a* | b* | |
| 0% | 53.11 ± 2.99 bc | 35.92 ± 3.93 a | 28.72 ± 1.84 d |
| 20% GLI | 53.86 ± 3.04 bc | 34.25 ± 2.75 ab | 29.87 ± 1.30 c |
| 30% GLI | 54.83 ± 2.44 b | 33.21 ± 2.62 b | 29.39 ± 1.91 cd |
| 20% SOR | 53.59 ± 2.08 bc | 34.74 ± 1.85 ab | 28.72 ± 1.03 d |
| 30% SOR | 52.89 ± 2.65 c | 35.34 ± 1.82 a | 29.21 ± 1.22 cd |
| 20% PEG | 61.84 ± 2.62 a | 17.94 ± 4.22 c | 34.45 ± 1.57 b |
| 30% PEG | 60.61 ± 2.08 a | 17.46 ± 1.72 c | 35.72 ± 1.31 a |
| Formulations | Contact Angle (°) | WVP (g.mm/m2.h.KPa) | Solubility (%) |
|---|---|---|---|
| 0% | 76.77 ± 2.37 a | 0.398 ± 0.043 e | 33.30 ± 4.94 d |
| 20% GLI | 66.44 ± 12.42 a | 0.610 ± 0.058 ab | 40.78 ± 2.34 abc |
| 30% GLI | 78.05 ± 2.52 a | 0.669 ± 0.076 a | 41.92 ± 1.11 ab |
| 20% SOR | 67.95 ± 1.58 a | 0.467 ± 0.020 de | 40.69 ± 0.65 abc |
| 30% SOR | 76.60 ± 5.10 a | 0.477 ± 0.022 de | 43.63 ± 0.49 a |
| 20% PEG | 67.96 ± 7.77 a | 0.507 ± 0.034 cd | 37.71 ± 2.11 c |
| 30% PEG | 49.96 ± 4.20 b | 0.574 ± 0.030 bc | 38.18 ± 1.41 bc |
| Formulations | Thickness (mm) | TR (MPa) | Elongation (%) |
|---|---|---|---|
| 0% | 0.158 ± 0.017 b | 3.28 ± 0.21 a | 45.87 ± 5.06 ab |
| 20% GLI | 0.160 ± 0.020 b | 2.31 ± 0.23 b | 42.25 ± 5.58 bc |
| 30% GLI | 0.160 ± 0.018 b | 1.83 ± 0.12 c | 40.88 ± 4.34 c |
| 20% SOR | 0.163 ± 0.020 ab | 2.46 ± 0.37 b | 43.43 ± 5.33 abc |
| 30% SOR | 0.163 ± 0.019 ab | 2.39 ± 0.25 b | 48.08 ± 3.66 a |
| 20% PEG | 0.161 ± 0.021 b | 3.17 ± 0.41 a | 46.44 ± 4.79 ab |
| 30% PEG | 0.171 ± 0.018 a | 2.42 ± 0.28 b | 32.75 ± 4.16 d |
| Formulations | Phenolic Compounds 1 | Flavonoids 2 | Anthocyanins 3 |
|---|---|---|---|
| 0% | 1194.55 ± 77.64 a | 48.88 ± 1.11 a | 31.72 ± 3.94 a |
| 20% SOR | 1115.47 ± 67.89 b | 47.70 ± 2.85 a | 29.06 ± 3.45 a |
| 30% SOR | 1042.10 ± 58.69 c | 46.91 ± 3.79 a | 28.95 ± 1.01 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freitas, T.S.M.d.; Garcia, V.A.d.S.; Filgueiras, C.T.; Velasco, J.I.; Fakhouri, F.M. Production of Edible Films Based on Pea Starch with Incorporation of Active Compounds Obtained from the Purple Araçá (Psidium myrtoides). Polymers 2021, 13, 3134. https://doi.org/10.3390/polym13183134
Freitas TSMd, Garcia VAdS, Filgueiras CT, Velasco JI, Fakhouri FM. Production of Edible Films Based on Pea Starch with Incorporation of Active Compounds Obtained from the Purple Araçá (Psidium myrtoides). Polymers. 2021; 13(18):3134. https://doi.org/10.3390/polym13183134
Chicago/Turabian StyleFreitas, Thainá Stéphanie Martins de, Vitor Augusto dos Santos Garcia, Cristina Tostes Filgueiras, José Ignacio Velasco, and Farayde Matta Fakhouri. 2021. "Production of Edible Films Based on Pea Starch with Incorporation of Active Compounds Obtained from the Purple Araçá (Psidium myrtoides)" Polymers 13, no. 18: 3134. https://doi.org/10.3390/polym13183134