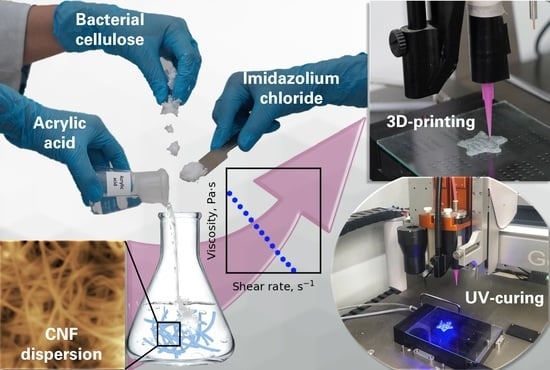
Polymerizable Choline- and Imidazolium-Based Ionic Liquids Reinforced with Bacterial Cellulose for 3D-Printing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Bacterial Synthesis of Cellulose
2.2.2. Preparation of Dispersions and Regeneration of Cellulose
2.2.3. Microscopic Investigation
2.2.4. Wide-Angle X-ray Diffraction Study
2.2.5. Fourier Transform Infrared Spectroscopy
2.2.6. Rheological Studies
2.2.7. Polymerization in Bulk and 3D-Printing
2.2.8. Measurements of Mechanical Properties
3. Results
3.1. Microscopy Investigation
3.2. WAXD Study
3.3. Fourier Transform Infrared Spectra
3.4. Rheological Measurements
3.5. 3D-Printing and Mechanical Properties
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, N.; Qiao, D.; Zhao, S.; Lin, Q.; Zhang, B.; Xie, F. 3D printing to innovate biopolymer materials for demanding applications: A review. Mater. Today Chem. 2021, 20, 100459. [Google Scholar] [CrossRef]
- Wang, J.; Chiappone, A.; Roppolo, I.; Shao, F.; Fantino, E.; Lorusso, M.; Rentsch, D.; Dietliker, K.; Pirri, C.F.; Grützmacher, H. All-in-One Cellulose Nanocrystals for 3D Printing of Nanocomposite Hydrogels. Angew. Chemie Int. Ed. 2018, 57, 2353–2356. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Sun, J.; Yao, Q.; Ji, C.; Liu, J.; Zhu, Q. 3D printing with cellulose materials. Cellulose 2018, 25, 4275–4301. [Google Scholar] [CrossRef]
- Hausmann, M.K.; Rühs, P.A.; Siqueira, G.; Läuger, J.; Libanori, R.; Zimmermann, T.; Studart, A.R. Dynamics of Cellulose Nanocrystal Alignment during 3D Printing. ACS Nano 2018, 12, 6926–6937. [Google Scholar] [CrossRef]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef] [PubMed]
- Doench, I.; Tran, T.; David, L.; Montembault, A.; Viguier, E.; Gorzelanny, C.; Sudre, G.; Cachon, T.; Louback-Mohamed, M.; Horbelt, N.; et al. Cellulose Nanofiber-Reinforced Chitosan Hydrogel Composites for Intervertebral Disc Tissue Repair. Biomimetics 2019, 4, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghanbari, A.; Tabarsa, T.; Ashori, A.; Shakeri, A.; Mashkour, M. Thermoplastic starch foamed composites reinforced with cellulose nanofibers: Thermal and mechanical properties. Carbohydr. Polym. 2018, 197, 305–311. [Google Scholar] [CrossRef]
- Tarrés, Q.; Oliver-Ortega, H.; Alcalà, M.; Espinach, X.X.; Mutjé, P.; Delgado-Aguilar, M. Research on the strengthening advantages on using cellulose nanofibers as polyvinyl alcohol reinforcement. Polymers 2020, 12, 974. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Huang, C.; Zhao, H.; Wang, J.; Yin, C.; Zhang, L.; Zhao, Y. Effects of cellulose nanocrystals and cellulose nanofibers on the structure and properties of polyhydroxybutyrate nanocomposites. Polymers 2019, 11, 2063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stepanova, M.; Averianov, I.; Serdobintsev, M.; Gofman, I.; Blum, N.; Semenova, N.; Nashchekina, Y.; Vinogradova, T.; Korzhikov-Vlakh, V.; Karttunen, M.; et al. PGlu-modified nanocrystalline cellulose improves mechanical properties, biocompatibility, and mineralization of polyester-based composites. Materials 2019, 12, 3435. [Google Scholar] [CrossRef] [Green Version]
- Yao, J.; Chen, S.; Chen, Y.; Wang, B.; Pei, Q.; Wang, H. Macrofibers with High Mechanical Performance Based on Aligned Bacterial Cellulose Nanofibers. ACS Appl. Mater. Interfaces 2017, 9, 20330–20339. [Google Scholar] [CrossRef]
- Svensson, A.; Nicklasson, E.; Harrah, T.; Panilaitis, B.; Kaplan, D.L.; Brittberg, M.; Gatenholm, P. Bacterial cellulose as a potential scaffold for tissue engineering of cartilage. Biomaterials 2005, 26, 419–431. [Google Scholar] [CrossRef]
- Buyanov, A.L.; Gofman, I.V.; Saprykina, N.N. High-strength cellulose–polyacrylamide hydrogels: Mechanical behavior and structure depending on the type of cellulose. J. Mech. Behav. Biomed. Mater. 2019, 100, 103385. [Google Scholar] [CrossRef] [Green Version]
- Sheykhnazari, S.; Tabarsa, T.; Mashkour, M.; Khazaeian, A.; Ghanbari, A. Multilayer bacterial cellulose/resole nanocomposites: Relationship between structural and electro-thermo-mechanical properties. Int. J. Biol. Macromol. 2018, 120, 2115–2122. [Google Scholar] [CrossRef]
- Gurtovenko, A.A.; Mukhamadiarov, E.I.; Kostritskii, A.Y.; Karttunen, M. Phospholipid-Cellulose Interactions: Insight from Atomistic Computer Simulations for Understanding the Impact of Cellulose-Based Materials on Plasma Membranes. J. Phys. Chem. B 2018, 122, 9973–9981. [Google Scholar] [CrossRef]
- Gurtovenko, A.A.; Karttunen, M. How to control interactions of cellulose-based biomaterials with skin: The role of acidity in the contact area. Soft Matter 2021, 17, 6507–6518. [Google Scholar] [CrossRef]
- Arellano, I.H.J.; Guarino, J.G.; Paredes, F.U.; Arco, S.D. Thermal stability and moisture uptake of 1-alkyl-3-methylimidazolium bromide. J. Therm. Anal. Calorim. 2011, 103, 725–730. [Google Scholar] [CrossRef]
- Kasprzak, D.; Krystkowiak, E.; Stępniak, I.; Galiński, M. Dissolution of cellulose in novel carboxylate-based ionic liquids and dimethyl sulfoxide mixed solvents. Eur. Polym. J. 2019, 113, 89–97. [Google Scholar] [CrossRef]
- Isik, M.; Sardon, H.; Mecerreyes, D. Ionic liquids and cellulose: Dissolution, chemical modification and preparation of new cellulosic materials. Int. J. Mol. Sci. 2014, 15, 11922–11940. [Google Scholar] [CrossRef] [PubMed]
- Swatloski, R.P.; Spear, S.K.; Holbrey, J.D.; Rogers, R.D. Dissolution of cellose with ionic liquids. J. Am. Chem. Soc. 2002, 124, 4974–4975. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Wu, J.; Zhang, J.; Niu, Y.; Liu, C.Y.; He, J.; Zhang, J. Rheological properties of cellulose/ionic liquid/dimethylsulfoxide (DMSO) solutions. Polymer 2012, 53, 2524–2531. [Google Scholar] [CrossRef]
- Morais, E.S.; Da Costa Lopes, A.M.; Freire, M.G.; Freire, C.S.R.; Coutinho, J.A.P.; Silvestre, A.J.D. Use of ionic liquids and deep eutectic solvents in polysaccharides dissolution and extraction processes towards sustainable biomass valorization. Molecules 2020, 25, 3652. [Google Scholar] [CrossRef]
- Hou, Q.; Ju, M.; Li, W.; Liu, L.; Chen, Y.; Yang, Q.; Zhao, H. Pretreatment of lignocellulosic biomass with ionic liquids and ionic liquid-based solvent systems. Molecules 2017, 22, 490. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wu, J.; Yu, J.; Zhang, X.; He, J.; Zhang, J. Application of ionic liquids for dissolving cellulose and fabricating cellulose-based materials: State of the art and future trends. Mater. Chem. Front. 2017, 1, 1273–1290. [Google Scholar] [CrossRef]
- Glińska, K.; Gitalt, J.; Torrens, E.; Plechkova, N.; Bengoa, C. Extraction of cellulose from corn stover using designed ionic liquids with improved reusing capabilities. Process Saf. Environ. Prot. 2021, 147, 181–191. [Google Scholar] [CrossRef]
- Magalhães Da Silva, S.P.; Da Costa Lopes, A.M.; Roseiro, L.B.; Bogel-Łukasik, R. Novel pre-treatment and fractionation method for lignocellulosic biomass using ionic liquids. RSC Adv. 2013, 3, 16040–16050. [Google Scholar] [CrossRef] [Green Version]
- Prasad, K.; Mine, S.; Kaneko, Y.; Kadokawa, J.I. Preparation of cellulose-based ionic porous material compatibilized with polymeric ionic liquid. Polym. Bull. 2010, 64, 341–349. [Google Scholar] [CrossRef]
- Chiappe, C.; Rodriguez Douton, M.J.; Mezzetta, A.; Pomelli, C.S.; Assanelli, G.; De Angelis, A.R. Recycle and Extraction: Cornerstones for an Efficient Conversion of Cellulose into 5-Hydroxymethylfurfural in Ionic Liquids. ACS Sustain. Chem. Eng. 2017, 5, 5529–5536. [Google Scholar] [CrossRef]
- Murakami, M.; Kaneko, Y.; Kadokawa, J. Preparation of cellulose-polymerized ionic liquid composite by in-situ polymerization of polymerizable ionic liquid in cellulose-dissolving solution. Carbohydr. Polym. 2007, 69, 378–381. [Google Scholar] [CrossRef]
- Isik, M.; Gracia, R.; Kollnus, L.C.; Tomé, L.C.; Marrucho, I.M.; Mecerreyes, D. Cholinium-based poly(ionic liquid)s: Synthesis, characterization, and application as biocompatible ion gels and cellulose coatings. ACS Macro Lett. 2013, 2, 975–979. [Google Scholar] [CrossRef]
- Ma, T.; Lv, L.; Ouyang, C.; Hu, X.; Liao, X.; Song, Y.; Hu, X. Rheological behavior and particle alignment of cellulose nanocrystal and its composite hydrogels during 3D printing. Carbohydr. Polym. 2021, 253, 117217. [Google Scholar] [CrossRef]
- Long, W.J.; Tao, J.L.; Lin, C.; Gu, Y.C.; Mei, L.; Duan, H.B.; Xing, F. Rheology and buildability of sustainable cement-based composites containing micro-crystalline cellulose for 3D-printing. J. Clean. Prod. 2019, 239, 118054. [Google Scholar] [CrossRef]
- Shin, S.; Hyun, J. Rheological properties of cellulose nanofiber hydrogel for high-fidelity 3D printing. Carbohydr. Polym. 2021, 263, 117976. [Google Scholar] [CrossRef]
- Shao, Y.; Chaussy, D.; Grosseau, P.; Beneventi, D. Use of Microfibrillated Cellulose/Lignosulfonate Blends as Carbon Precursors: Impact of Hydrogel Rheology on 3D Printing. Ind. Eng. Chem. Res. 2015, 54, 10575–10582. [Google Scholar] [CrossRef]
- Song, H.; Zhang, J.; Niu, Y.; Wang, Z. Phase transition and rheological behaviors of concentrated cellulose/ionic liquid solutions. J. Phys. Chem. B 2010, 114, 6006–6013. [Google Scholar] [CrossRef] [PubMed]
- Sescousse, R.; Le, K.A.; Ries, M.E.; Budtova, T. Viscosity of cellulose-imidazolium-based ionic liquid solutions. J. Phys. Chem. B 2010, 114, 7222–7228. [Google Scholar] [CrossRef]
- Rudaz, C.; Budtova, T. Rheological and hydrodynamic properties of cellulose acetate/ionic liquid solutions. Carbohydr. Polym. 2013, 92, 1966–1971. [Google Scholar] [CrossRef]
- Gericke, M.; Schlufter, K.; Liebert, T.; Heinze, T.; Budtova, T. Rheological properties of cellulose/ionic liquid solutions: From dilute to concentrated states. Biomacromolecules 2009, 10, 1188–1194. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Sun, N.; He, X.; Lu, X.; Zhang, X. Physical properties of ionic liquids: Database and evaluation. J. Phys. Chem. Ref. Data 2006, 35, 1475–1517. [Google Scholar] [CrossRef]
- Hapiot, P.; Lagrost, C. Electrochemical reactivity in room-temperature ionic liquids. Chem. Rev. 2008, 108, 2238–2264. [Google Scholar] [CrossRef]
- Fendt, S.; Padmanabhan, S.; Blanch, H.W.; Prausnitz, J.M. Viscosities of acetate or chloride-based ionic liquids and some of their mixtures with water or other common solvents. J. Chem. Eng. Data 2011, 56, 31–34. [Google Scholar] [CrossRef]
- Yang, F.; Wang, X.; Tan, H.; Liu, Z. Improvement the viscosity of imidazolium-based ionic liquid using organic solvents for biofuels. J. Mol. Liq. 2017, 248, 626–633. [Google Scholar] [CrossRef]
- Gunasekera, D.H.A.T.; Kuek, S.; Hasanaj, D.; He, Y.; Tuck, C.; Croft, A.K.; Wildman, R.D. Three dimensional ink-jet printing of biomaterials using ionic liquids and co-solvents. Faraday Discuss. 2016, 190, 509–523. [Google Scholar] [CrossRef] [Green Version]
- Amde, M.; Liu, J.-F.; Pang, L. Environmental application, fate, effects, and concerns of ionic liquids: A review. Environ. Sci. Technol. 2015, 49, 12611–12627. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [Green Version]
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W.; et al. Deep Eutectic Solvents: A Review of Fundamentals and Applications. Chem. Rev. 2021, 121, 1232–1285. [Google Scholar] [CrossRef]
- Gurkan, B.E.; Maginn, E.J.; Pentzer, E.B. Deep eutectic solvents: A new class of versatile liquids. J. Phys. Chem. B 2020, 124, 11313–11315. [Google Scholar] [CrossRef]
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural deep eutectic solvents—Solvents for the 21st century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071. [Google Scholar] [CrossRef]
- Galvis-Sánchez, A.C.; Castro, M.C.R.; Biernacki, K.; Gonçalves, M.P.; Souza, H.K.S. Natural deep eutectic solvents as green plasticizers for chitosan thermoplastic production with controlled/desired mechanical and barrier properties. Food Hydrocoll. 2018, 82, 478–489. [Google Scholar] [CrossRef]
- Sokolova, M.P.; Smirnov, M.A.; Samarov, A.A.; Bobrova, N.V.; Vorobiov, V.K.; Popova, E.N.; Filippova, E.; Geydt, P.; Lahderanta, E.; Toikka, A.M. Plasticizing of chitosan films with deep eutectic mixture of malonic acid and choline chloride. Carbohydr. Polym. 2018, 197, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Smirnov, M.A.; Nikolaeva, A.L.; Bobrova, N.V.; Vorobiov, V.K.; Smirnov, A.V.; Lahderanta, E.; Sokolova, M.P. Self-healing films based on chitosan containing citric acid/choline chloride deep eutectic solvent. Polym. Test. 2021, 97, 107156. [Google Scholar] [CrossRef]
- Xie, Q.; Zheng, X.; Li, L.; Ma, L.; Zhao, Q.; Chang, S.; You, L. Effect of Curcumin Addition on the Properties of Biodegradable Pectin/Chitosan Films. Molecules 2021, 26, 2152. [Google Scholar] [CrossRef] [PubMed]
- Jakubowska, E.; Gierszewska, M.; Nowaczyk, J.; Olewnik-Kruszkowska, E. The role of a deep eutectic solvent in changes of physicochemical and antioxidative properties of chitosan-based films. Carbohydr. Polym. 2021, 255, 117527. [Google Scholar] [CrossRef] [PubMed]
- Zdanowicz, M. Deep eutectic solvents based on urea, polyols and sugars for starch treatment. Int. J. Biol. Macromol. 2021, 176, 387–393. [Google Scholar] [CrossRef]
- Özel, N.; Elibol, M. A review on the potential uses of deep eutectic solvents in chitin and chitosan related processes. Carbohydr. Polym. 2021, 262, 117942. [Google Scholar] [CrossRef]
- Yuan, Y.; Hong, S.; Lian, H.; Zhang, K.; Liimatainen, H. Comparison of acidic deep eutectic solvents in production of chitin nanocrystals. Carbohydr. Polym. 2020, 236, 116095. [Google Scholar] [CrossRef]
- Mukesh, C.; Mondal, D.; Sharma, M.; Prasad, K. Choline chloride-thiourea, a deep eutectic solvent for the production of chitin nanofibers. Carbohydr. Polym. 2014, 103, 466–471. [Google Scholar] [CrossRef]
- Smirnov, M.A.; Sokolova, M.P.; Tolmachev, D.A.; Vorobiov, V.K.; Kasatkin, I.A.; Smirnov, N.N.; Klaving, A.V.; Bobrova, N.V.; Lukasheva, N.V.; Yakimansky, A.V. Green method for preparation of cellulose nanocrystals using deep eutectic solvent. Cellulose 2020, 27, 4305–4317. [Google Scholar] [CrossRef]
- Jiang, J.; Zhu, Y.; Jiang, F. Sustainable isolation of nanocellulose from cellulose and lignocellulosic feedstocks: Recent progress and perspectives Jungang. Carbohydr. Polym. 2021, 118188. [Google Scholar] [CrossRef]
- Sirviö, J.A.; Hyypiö, K.; Asaadi, S.; Junka, K.; Liimatainen, H. High-strength cellulose nanofibers produced: Via swelling pretreatment based on a choline chloride-imidazole deep eutectic solvent. Green Chem. 2020, 22, 1763–1775. [Google Scholar] [CrossRef] [Green Version]
- Sirviö, J.A.; Visanko, M.; Liimatainen, H. Deep eutectic solvent system based on choline chloride-urea as a pre-treatment for nanofibrillation of wood cellulose. Green Chem. 2015, 17, 3401–3406. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, B.; Xia, Q.; Meng, J.; Chen, W.; Liu, S.; Wang, Q.; Liu, Y.; Li, J.; Yu, H. Efficient Cleavage of Strong Hydrogen Bonds in Cotton by Deep Eutectic Solvents and Facile Fabrication of Cellulose Nanocrystals in High Yields. ACS Sustain. Chem. Eng. 2017, 5, 7623–7631. [Google Scholar] [CrossRef]
- Fan, Q.; Jiang, C.; Wang, W.; Bai, L.; Chen, H.; Yang, H.; Wei, D.; Yang, L. Eco-friendly extraction of cellulose nanocrystals from grape pomace and construction of self-healing nanocomposite hydrogels. Cellulose 2020, 27, 2541–2553. [Google Scholar] [CrossRef]
- Li, C.; Huang, C.; Zhao, Y.; Zheng, C.; Su, H.; Zhang, L.; Luo, W.; Zhao, H.; Wang, S.; Huang, L.J. Effect of choline-based deep eutectic solvent pretreatment on the structure of cellulose and lignin in Bagasse. Processes 2021, 9, 384. [Google Scholar] [CrossRef]
- Jablonsky, M.; Haz, A.; Majova, V. Assessing the opportunities for applying deep eutectic solvents for fractionation of beech wood and wheat straw. Cellulose 2019, 26, 7675–7684. [Google Scholar] [CrossRef]
- Fazende, K.F.; Phachansitthi, M.; Mota-Morales, J.D.; Pojman, J.A. Frontal Polymerization of Deep Eutectic Solvents Composed of Acrylic and Methacrylic Acids. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 4046–4050. [Google Scholar] [CrossRef]
- Mota-Morales, J.D.; Sánchez-Leija, R.J.; Carranza, A.; Pojman, J.A.; del Monte, F.; Luna-Bárcenas, G. Free-radical polymerizations of and in deep eutectic solvents: Green synthesis of functional materials. Prog. Polym. Sci. 2018, 78, 139–153. [Google Scholar] [CrossRef]
- Yue, C.; Li, M.; Liu, Y.; Fang, Y.; Song, Y.; Xu, M.; Li, J. Three-dimensional Printing of Cellulose Nanofibers Reinforced PHB/PCL/Fe3O4 Magneto-responsive Shape Memory Polymer Composites with Excellent Mechanical Properties. Addit. Manuf. 2021, 102146. [Google Scholar] [CrossRef]
- Li, V.C.F.; Kuang, X.; Mulyadi, A.; Hamel, C.M.; Deng, Y.; Qi, H.J. 3D printed cellulose nanocrystal composites through digital light processing. Cellulose 2019, 26, 3973–3985. [Google Scholar] [CrossRef]
- Li, V.C.F.; Dunn, C.K.; Zhang, Z.; Deng, Y.; Qi, H.J. Direct Ink Write (DIW) 3D Printed Cellulose Nanocrystal Aerogel Structures. Sci. Rep. 2017, 7, 8018. [Google Scholar] [CrossRef] [PubMed]
- Kam, D.; Braner, A.; Abouzglo, A.; Larush, L.; Chiappone, A.; Shoseyov, O.; Magdassi, S. 3D Printing of Cellulose Nanocrystal-Loaded Hydrogels through Rapid Fixation by Photopolymerization. Langmuir 2021, 37, 6451–6458. [Google Scholar] [CrossRef]
- Melilli, G.; Carmagnola, I.; Tonda-Turo, C.; Pirri, F.; Ciardelli, G.; Sangermano, M.; Hakkarainen, M.; Chiappone, A. DLP 3D printing meets lignocellulosic biopolymers: Carboxymethyl cellulose inks for 3D biocompatible hydrogels. Polymers 2020, 12, 12081655. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, G.; Kokkinis, D.; Libanori, R.; Hausmann, M.K.; Gladman, A.S.; Neels, A.; Tingaut, P.; Zimmermann, T.; Lewis, J.A.; Studart, A.R. Cellulose Nanocrystal Inks for 3D Printing of Textured Cellular Architectures. Adv. Funct. Mater. 2017, 27, 1604619. [Google Scholar] [CrossRef]
- Lai, P.C.; Yu, S.S. Cationic cellulose nanocrystals-based nanocomposite hydrogels: Achieving 3d printable capacitive sensors with high transparency and mechanical strength. Polymers 2021, 13, 688. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.W.; Yu, S.S. 3D Printable Strain Sensors from Deep Eutectic Solvents and Cellulose Nanocrystals. ACS Appl. Mater. Interfaces 2020, 12, 34235–34244. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.; Kawakami, M.; Khosla, A.; Furukawa, H. Soft, conductive nanocomposites based on ionic liquids/carbon nanotubes for 3D printing of flexible electronic devices. Polym. J. 2019, 51, 511–521. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, B.; Zhang, Y.; Cao, Q.; Yang, C.; Li, Y.; Zhou, J. Biomimetic lignin/poly(ionic liquids) composite hydrogel dressing with excellent mechanical strength, self-healing properties, and reusability. Chem. Eng. J. 2020, 400, 125984. [Google Scholar] [CrossRef]
- Zhu, M.; Cao, Q.; Liu, B.; Guo, H.; Wang, X.; Han, Y.; Sun, G.; Li, Y.; Zhou, J. A novel cellulose acetate/poly (ionic liquid) composite air filter. Cellulose 2020, 27, 3889–3902. [Google Scholar] [CrossRef]
- Smirnov, M.A.; Vorobiov, V.K.; Bobrova, N.V.; Smirnov, N.N.; Lyulin, S.V. METHOD OF PRODUCING BACTERIAL CELLULOSE NANOFIBERS RU. In Proceedings of the 15 th International Saint Petersburg Conference of Young Scientists, Saint Petersburg, Russia, 28–31 October 2019; Institute of Macromolecular Compounds of the Russian Academy of Sciences Saint Petersburg: Sant Petersburg, Russia, 2019; pp. 1–8. [Google Scholar]
- Xu, J.; Zhang, B.; Lu, X.; Zhou, Y.; Fang, J.; Li, Y.; Zhang, S. Nanoscale Observation of Microfibril Swelling and Dissolution in Ionic Liquids. ACS Sustain. Chem. Eng. 2018, 6, 909–917. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-Ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Dong, J.; Ozaki, Y.; Nakashima, K. Infrared, Raman, and near-infrared spectroscopic evidence for the coexistence of various hydrogen-bond forms in poly(acrylic acid). Macromolecules 1997, 30, 1111–1117. [Google Scholar] [CrossRef]
- Czarnecka, E.; Nowaczyk, J. Semi-Natural superabsorbents based on Starch-g-poly(acrylic acid): Modification, synthesis and application. Polymers 2020, 12, 1794. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Chen, C.; Hou, Z.; Wei, H. Poly (acrylic acid sodium) grafted carboxymethyl cellulose as a high performance polymer binder for silicon anode in lithium ion batteries. Sci. Rep. 2016, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hon, N.S. Formation of Free Radicals in Photoirradiated Cellulose—8. Mechanisms. J. Polym. Sci. Polym. Chem. Ed. 1976, 14, 2497–2512. [Google Scholar] [CrossRef]
- Fazende, K.F.; Gary, D.P.; Mota-Morales, J.D.; Pojman, J.A. Kinetic Studies of Photopolymerization of Monomer-Containing Deep Eutectic Solvents. Macromol. Chem. Phys. 2020, 221, 1900511. [Google Scholar] [CrossRef]
- Okoturo, O.O.; VanderNoot, T.J. Temperature dependence of viscosity for room temperature ionic liquids. J. Electroanal. Chem. 2004, 568, 167–181. [Google Scholar] [CrossRef]
- Hammond, O.S.; Bowron, D.T.; Edler, K.J. Liquid structure of the choline chloride-urea deep eutectic solvent (reline) from neutron diffraction and atomistic modelling. Green Chem. 2016, 18, 2736–2744. [Google Scholar] [CrossRef] [Green Version]
- Gontrani, L.; Bonomo, M.; Plechkova, N.V.; Dini, D.; Caminiti, R. X-Ray structure and ionic conductivity studies of anhydrous and hydrated choline chloride and oxalic acid deep eutectic solvents. Phys. Chem. Chem. Phys. 2018, 20, 30120–30124. [Google Scholar] [CrossRef]
- Stassen, H.K.; Ludwig, R.; Wulf, A.; Dupont, J. Imidazolium salt ion Pairs in solution. Chem. A Eur. J. 2015, 21, 8324–8335. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Z.; Han, B.; Hu, S.; Xie, Y.; Yang, G. Effect of water and organic solvents on-the ionic dissociation of ionic liquids. J. Phys. Chem. B 2007, 111, 6452–6456. [Google Scholar] [CrossRef]
- Czaikoski, A.; da Cunha, R.L.; Menegalli, F.C. Rheological behavior of cellulose nanofibers from cassava peel obtained by combination of chemical and physical processes. Carbohydr. Polym. 2020, 248, 116744. [Google Scholar] [CrossRef] [PubMed]
- Barnes, H.A. A review of the slip (wall depletion) of polymer solutions, emulsions and particle suspensions in viscometers: Its cause, character, and cure. J. Nonnewton. Fluid Mech. 1995, 56, 221–251. [Google Scholar] [CrossRef]
- Reid, M.S.; Kedzior, S.A.; Villalobos, M.; Cranston, E.D. Effect of Ionic Strength and Surface Charge Density on the Kinetics of Cellulose Nanocrystal Thin Film Swelling. Langmuir 2017, 33, 7403–7411. [Google Scholar] [CrossRef]
- Wang, H.; Gurau, G.; Rogers, R.D. Ionic liquid processing of cellulose. Chem. Soc. Rev. 2012, 41, 1519–1537. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, J.; Liu, X.; Zhang, S. Towards a molecular understanding of cellulose dissolution in ionic liquids: Anion/cation effect, synergistic mechanism and physicochemical aspects. Chem. Sci. 2018, 9, 4027–4043. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Liu, X.; Zhang, Y.; Jiang, K.; Wang, J.; Zhang, S. Why Only Ionic Liquids with Unsaturated Heterocyclic Cations Can Dissolve Cellulose: A Simulation Study. ACS Sustain. Chem. Eng. 2017, 5, 3417–3428. [Google Scholar] [CrossRef]
- Hong, S.; Yuan, Y.; Liu, C.; Chen, W.; Chen, L.; Lian, H.; Liimatainen, H. A stretchable and compressible ion gel based on a deep eutectic solvent applied as a strain sensor and electrolyte for supercapacitors. J. Mater. Chem. C 2020, 8, 550–560. [Google Scholar] [CrossRef]










| Sample | E10–15%, MPa | σ50%, MPa | σ80%, MPa |
|---|---|---|---|
| AA/ImCl-based ion gel | 2.1 ± 0.2 | 2.7 ± 0.3 | 45 ± 15 |
| AA/ChCl-based ion gel | 6.8 ± 0.9 | 5.7 ± 0.4 | 49 ± 6 |
| Sample | E, MPa | σb, MPa | εb, % |
|---|---|---|---|
| AA/ImCl-based ion gel | 0.51 ± 0.05 | 0.38 ± 0.05 | 94 ± 14 |
| AA/ChCl-based ion gel | 0.56 ± 0.06 | 0.56 ± 0.02 | 175 ± 25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smirnov, M.A.; Fedotova, V.S.; Sokolova, M.P.; Nikolaeva, A.L.; Elokhovsky, V.Y.; Karttunen, M. Polymerizable Choline- and Imidazolium-Based Ionic Liquids Reinforced with Bacterial Cellulose for 3D-Printing. Polymers 2021, 13, 3044. https://doi.org/10.3390/polym13183044
Smirnov MA, Fedotova VS, Sokolova MP, Nikolaeva AL, Elokhovsky VY, Karttunen M. Polymerizable Choline- and Imidazolium-Based Ionic Liquids Reinforced with Bacterial Cellulose for 3D-Printing. Polymers. 2021; 13(18):3044. https://doi.org/10.3390/polym13183044
Chicago/Turabian StyleSmirnov, Michael A., Veronika S. Fedotova, Maria P. Sokolova, Alexandra L. Nikolaeva, Vladimir Yu. Elokhovsky, and Mikko Karttunen. 2021. "Polymerizable Choline- and Imidazolium-Based Ionic Liquids Reinforced with Bacterial Cellulose for 3D-Printing" Polymers 13, no. 18: 3044. https://doi.org/10.3390/polym13183044