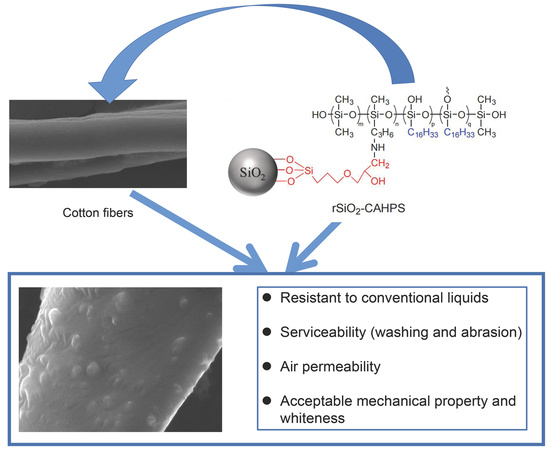
Preparation and Application of Fluorine-Free Finishing Agent with Excellent Water Repellency for Cotton Fabric
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation Procedures
2.2.1. Synthesis of Polysiloxane
2.2.2. Modification of Silica
2.2.3. Synthesis of rSiO2–CAHPS
2.2.4. Preparation of Fluorine-Free Water Repellent Agent
2.3. Finishing with rSiO2–CAHPS Water Repellent
2.4. Characterization Methods
3. Results and Discussion
3.1. Preparation of Fluorine-Free Water Repellent Agent
3.2. Characterization and Analysis of Cotton Fabric Surface
3.2.1. Surface Morphology of Cotton Fibers
3.2.2. Elemental Analysis of the Cotton Fiber Surface
3.3. Performance Evaluation of Cotton Fabric after Finishing
3.3.1. Water Repellency
3.3.2. Air Permeability of Finished Cotton Fabric
3.3.3. Serviceability of Finished Cotton Fabric
3.3.4. Performance Comparison with Commercially Available Water Repellent
3.4. Proposal of the Film Formation Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xue, C.H.; Zhang, L.; Wei, P.; Wei, P.B.; Jia, S.T. Fabrication of superhydrophobic cotton textiles with flame retardancy. Cellulose 2016, 23, 1471–1480. [Google Scholar] [CrossRef]
- Zhong, Y.D.; Netravali, A.N. ‘Green’ surface treatment for water-repellent cotton fabrics. Surf. Innov. 2016, 4, 3–13. [Google Scholar] [CrossRef]
- Boukhriss, A.; Boyer, D.; Hannache, H.; Roblin, J.P.; Mahiou, R.; Cherkaoui, O.; Therias, S.; Gmouh, S. Sol-gel based water repellent coatings for textiles. Cellulose 2015, 22, 1415–1425. [Google Scholar] [CrossRef]
- Ma, Y.; Zhu, D.J.; Si, Y.; Sun, G. Fabricating durable, fluoride-free, water repellency cotton fabrics with CPDMS. J. Appl. Polym. Sci. 2018, 135, 46396. [Google Scholar] [CrossRef]
- Guo, F.; Fang, K.; Song, Y.; Fu, R.; Li, H.; Zhang, C. Optimizing a fabricating program for wearable super-hydrophobic cotton by clean production technology of plasma and reducing chemical consumption. J. Clean. Prod. 2020, 276, 124233. [Google Scholar] [CrossRef]
- Xue, C.H.; Du, M.M.; Guo, X.J.; Liu, B.Y.; Wei, R.X.; Li, H.G.; Huang, M.C.; Deng, F.Q.; Jia, S.T. Fabrication of superhydrophobic photothermal conversion fabric via layer-by-layer assembly of carbon nanotubes. Cellulose 2021, 28, 5107–5121. [Google Scholar] [CrossRef]
- Jiang, C.; Liu, W.; Yang, M.P.; He, S.; Xie, Y.K.; Wang, Z.F. Synthesis of superhydrophobic fluoro-containing silica sol coatings for cotton textile by one-step sol-gel process. J. Sol-Gel Sci. Technol. 2018, 87, 455–463. [Google Scholar] [CrossRef]
- Zhang, H.; Hou, C.P.; Song, L.X.; Ma, Y.; Ali, Z.; Gu, J.W.; Zhang, B.L.; Zhang, H.P.; Zhang, Q.Y. A stable 3D sol-gel network with dangling fluoroalkyl chains and rapid self-healing ability as a long-lived superhydrophobic fabric coating. Chem. Eng. J. 2018, 334, 598–610. [Google Scholar] [CrossRef]
- Henner, C. PFOA called likely human carcinogen. Chem. Eng. News 2005, 83, 5–16. [Google Scholar]
- Rebecca, R. PFOS phaseout pays off. Environ. Sci. Technol. 2008, 42, 4618. [Google Scholar]
- Zhou, Q.; Chen, G.; Xing, T. Facile construction of robust superhydrophobic tea polyphenol/Fe@cotton fabric for self-cleaning and efficient oil-water separation. Cellulose 2018, 25, 1513–1525. [Google Scholar] [CrossRef]
- Kaerrman, A.; Langlois, I.; Bavel, B.V.; Lindstrom, G.; Oehme, M. Identification and pattern of perfluorooctane sulfonate (PFOS) isomers in human serum and plasma. Environ. Int. 2007, 33, 782–788. [Google Scholar] [CrossRef] [PubMed]
- Russell, M.H.; Nilsson, H.; Buck, R.C. Elimination kinetics of perfluorohexanoic acid in humans and comparison with mouse, rat and monkey. Chemosphere 2013, 93, 2419–2425. [Google Scholar] [CrossRef]
- Mai, Z.; Shu, X.; Li, G.; Chen, D.Z.; Liu, M.; Xu, W.L.; Zhang, H.W. One-step fabrication of flexible, durable and fluorine-free superhydrophobic cotton fabrics for efficient oil/water separation. Cellulose 2019, 26, 6349–6363. [Google Scholar] [CrossRef]
- Hao, L.F.; An, Q.F.; Wei, X. Facile fabrication of superhydrophobic cotton fabric from stearyl methacrylate modified polysiloxane/silica nanocomposite. Fiber. Polym. 2012, 13, 1145–1153. [Google Scholar] [CrossRef]
- Ansari, A.; Trehan, R.; Watson, C.; Senyo, S. Increasing silicone mold longevity: A review of surface modification techniques for PDMS-PDMS double casting. Soft Mater. 2020. [Google Scholar] [CrossRef]
- Yang, J.; Pu, Y.; Miao, D.G.; Ning, X. Fabrication of durably superhydrophobic cotton fabrics by atmospheric pressure plasma treatment with a siloxane precursor. Polymers 2018, 10, 460. [Google Scholar] [CrossRef] [Green Version]
- Kasapgil, E.; Anac, I.; Erbil, H.Y. Transparent, fluorine-free, heat-resistant, water repellent coating by infusing slippery silicone oil on polysiloxane nanofilament layers prepared by gas phase reaction of n-propyltrichlorosilane and methyltrichlorosilane. Collids Surf. A 2019, 560, 223–232. [Google Scholar] [CrossRef]
- Zheng, G.L.; Wu, Y.H.; Zhang, D.; Liu, S.; Li, R. Facile fabrication of non-fluorinated durable superhydrophobic cotton fabric. Fiber. Polym. 2020, 21, 2513–2520. [Google Scholar] [CrossRef]
- Pan, Y.; Liang, Q.Y.; Song, L.; Zhao, H.T. Fabrication of layer-by-layer self-assembled coating modified cotton fabric with flame retardancy and hydrophobicity based on sepiolite. Polym.-Plast. Tech. Mater. 2021, 60, 1368–1376. [Google Scholar] [CrossRef]
- Ou, J.; Wu, B.; Wang, F.; Xue, M. Textile with Janus wetting properties via copper deposition and subsequent chemical vapor deposition of 1-dodecanethiol. Mater. Lett. 2019, 251, 5–7. [Google Scholar] [CrossRef]
- Rabia, S.; Muhammad, M.; Naveed, R.; Shaheen, S.; Syed, W.A.; Wahla, A. Development of bio and non fluorinated palmitic acid based water repellent for cotton fabric. J. Nat. Fibers 2021. [Google Scholar] [CrossRef]
- Rabia, S.; Muhammad, M.; Naveed, R.; Syed, W.A.; Haji, G.Q. Synthesis and application of fluorine free environment friendly stearic acid based oil and water repellent for cotton fabric. J. Nat. Fibers 2020. [Google Scholar] [CrossRef]
- Wei, X.; Zhang, F. Surface and mechanical properties of an organic-inorganic superhydrophobic coating using modified nano-SiO2 and mixing polyurethane emulsion as raw materials. J. Adhes. Sci. Technol. 2018, 32, 1809–1821. [Google Scholar] [CrossRef]
- Kong, X.; Zhu, C.; Lv, J.; Zhang, J.; Feng, J. Robust fluorine-free superhydrophobic coating on polyester fabrics by spraying commercial adhesive and hydrophobic fumed SiO2 nanoparticles. Prog. Org. Coat. 2020, 138, 105342. [Google Scholar] [CrossRef]
- Heale, F.; Page, K.; Wixey, J.S.; Taylor, P.; Parkin, I.P.; Carmalt, C.J. Inexpensive and non-toxic water repellent coatings comprising SiO2 nanoparticles and long chain fatty acids. RSC Adv. 2018, 8, 27064–27072. [Google Scholar] [CrossRef] [Green Version]
- Shaban, M.; Mohamed, F.; Abdallah, S. Production and characterization of superhydrophobic and antibacterial coated fabrics utilizing ZnO nanocatalyst. Sci. Rep. 2018, 8, 3925–3939. [Google Scholar] [CrossRef]
- Yang, M.P.; Liu, W.Q.; Jiang, C.; Liu, C.H.; He, S.; Xie, Y.K.; Wang, Z.G. Robust fabrication of superhydrophobic and photocatalytic self-cleaning cotton textile based on TiO2 and fluoroalkylsilane. J. Mater. Sci. 2019, 54, 2079–2092. [Google Scholar] [CrossRef]
- He, T.J.; Liu, X.; Wang, Y.X.; Wu, D.F.; Liu, Y.; Liu, X.Y. Fabrication of durable hierarchical superhydrophobic fabrics with Sichuan pepper-like structures via graft precipitation polymerization. Appl. Surf. Sci. 2020, 529, 147017. [Google Scholar] [CrossRef]
- Kim, S.; Oh, J.H.; Park, C.H. Development of energy-efficient superhydrophobic polypropylene fabric by oxygen plasma etching and thermal aging. Polymers 2020, 12, 2756. [Google Scholar] [CrossRef]
- Wu, M.C.; An, N.; Li, Y.; Sun, J.Q. Layer-by-layer assembly of fluorine-free polyelectrolyte surfactant complexes for the fabrication of self-healing superhydrophobic films. Langmuir 2016, 32, 12361–12369. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Hu, C.C.; Zhu, Y.J.; Zhang, H.H.; Li, R.; Xing, Y.J. Multi-functional finishing of cotton fabrics by water-based layer-by-layer assembly of metal-organic framework. Cellulose 2018, 25, 4223–4238. [Google Scholar] [CrossRef]
- Cui, F.H.; Han, W.D.; Ge, J.L.; Wu, X.H.; Kim, H.Y.; Ding, B. Electrospinning: A versatile strategy for mimicking natural creatures. Compos. Commun. 2018, 10, 175–185. [Google Scholar] [CrossRef]
- Khan, A.; Habib, M.R.; Kumar, R.R.; Islam, S.M.; Arivazhagan, V.; Salman, M.; Yang, D.; Yu, X.G. Wetting behaviors and applications of metal-catalyzed CVD grown graphene. J. Mater. Chem. A 2008, 6, 22437–22464. [Google Scholar] [CrossRef]
- Pakpum, C.; Kanchiang, K. Fabrication of DLC nanoparticle clusters by μ-wave oven based plasma reactor with acetylene diluted in air precursor. Appl. Surf. Sci. 2018, 458, 100–110. [Google Scholar] [CrossRef]
- Doganci, M.D. Fabrication of superhydrophobic transparent cyclic olefin copolymer (COC)-SiO2 nanocomposite surfaces. J. Appl. Polym. Sci. 2021, 138, 50145. [Google Scholar] [CrossRef]
- Shi, H.; Wang, T.T.; Zhao, L.; Zhu, W.H. Engineering superhydrophobic-superoleophilic nylon mesh for high-efficiency oil-water separation, Desalin. Water Treat. 2020, 204, 438–448. [Google Scholar]
- Wu, D.H.; Gao, Z.W.; Gao, L.X. Preparation of β-Cyclodextrin Polymer/Titanium Dioxide Inorganic-Organic Hybrid Materials by Sol-gel Method. ACTA Chim. Sin. 2006, 64, 716–720. [Google Scholar]
- Cassie, A.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Su, B.; Ye, T.; Lei, J. Bioinspired interfaces with superwettability: From materials to chemistry. J. Am. Chem. Soc. 2016, 138, 1727–1748. [Google Scholar] [CrossRef]
- Balu, B.; Breedveld, V.; Hess, D.W. Fabrication of “roll-off” and “sticky” superhydrophobic cellulose surfaces via plasma processing. Langmuir 2008, 24, 4785–4790. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Guo, X.; Li, L.; Wu, S.L.; Chu, P.K.; Xu, Z.S. Preparation and characterization of fluorinated acrylate copolymer latexes by miniemulsion polymerization under microwave irradiation. J. Fluor. Chem. 2010, 131, 417–425. [Google Scholar] [CrossRef]
- Yu, C.B.; Liu, Y.Q.; Lu, Y.L.; Tao, K.X.; Yi, Z. A salt-free pad-irradiate-pad-steam reactive dyeing process for cotton fabric and the influence of cationising conditions on its coloration. Color. Technol. 2021, 137, 399–406. [Google Scholar] [CrossRef]









| Samples | Breaking Strength (N) | Whiteness (%) | |
|---|---|---|---|
| Warp | Weft | ||
| Unfinished fabric | 331 ± 10 | 151 ± 5 | 75.76 ± 1.26 |
| Finished fabric | 322 ± 9 | 146 ± 3 | 72.67 ± 1.19 |
| Rate of change (%) | −2.7 | −3.3 | −4.1% |
| Samples | WCA (°) | Air Permeability | Washing Cycles, WCA (°) | 20 Abrasion Cycles, WCA (°) |
|---|---|---|---|---|
| WP-107A | 147.1–148.9 | Good | N/A * (20 cycles) | 111.6–112.6 |
| rSiO2–CAHPS | 149.1–150.2 | Good | 142.8–143.4 (20 cycles) 140.6–141.1 (30 cycles) | 111.8–112.5 |
| Unfinished | N/A * | Good | N/A * | N/A * |
| Samples | Not Washing (g) | After Washing (g) |
|---|---|---|
| After drying | 105.992–106.008 | 99.992–100.004 |
| After steaming | 105.899–106.005 | 105.989–106.004 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, C.; Shi, K.; Ning, J.; Zheng, Z.; Yu, H.; Yang, Z.; Liu, J. Preparation and Application of Fluorine-Free Finishing Agent with Excellent Water Repellency for Cotton Fabric. Polymers 2021, 13, 2980. https://doi.org/10.3390/polym13172980
Yu C, Shi K, Ning J, Zheng Z, Yu H, Yang Z, Liu J. Preparation and Application of Fluorine-Free Finishing Agent with Excellent Water Repellency for Cotton Fabric. Polymers. 2021; 13(17):2980. https://doi.org/10.3390/polym13172980
Chicago/Turabian StyleYu, Chengbing, Kaiqin Shi, Jinyan Ning, Zhe Zheng, Hualong Yu, Zhenxuan Yang, and Jun Liu. 2021. "Preparation and Application of Fluorine-Free Finishing Agent with Excellent Water Repellency for Cotton Fabric" Polymers 13, no. 17: 2980. https://doi.org/10.3390/polym13172980