Improvement in Solubility and Absorption of Nifedipine Using Solid Solution: Correlations between Surface Free Energy and Drug Dissolution
Abstract
:1. Introduction
2. Material and Methods
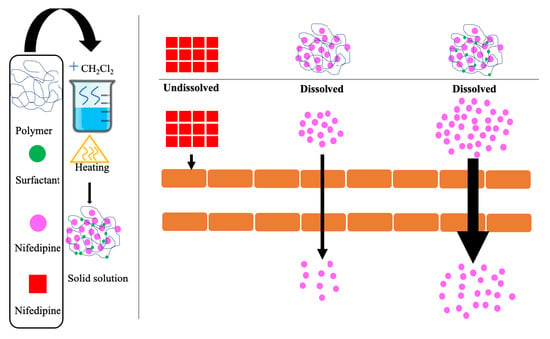
2.1. Preparation of Solid Solutions
2.2. Analysis of NDP
2.3. Morphological Examination
2.4. PXRD
2.5. DSC
2.6. Fourier-Transform Infrared Spectroscopy (FTIR)
2.7. Hot Stage Microscopy
2.8. Surface Free Energy Determination
2.9. In Vitro Dissolution Study
2.10. Evaluating Cellular Uptake of NDP
2.11. Stability of NDP
2.12. Statistical Analysis
3. Results and Discussion
3.1. Physical Properties of NDP Solid Solution
3.2. Dissolution of NDP Solid Solution
3.3. Evaluating Cellular Uptake of NDP
3.4. Stability of Solid Solution after Storage
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haware, R.V.; Vinjamuri, B.P.; Gavireddi, M.; Dave, V.S.; Gupta, D.; Chougule, M.B.; Stagner, W.C. Physical Properties and Solubility Studies of Nifedipine-PEG 1450/HPMCAS-HF Solid Dispersions. Pharm. Dev. Technol. 2019, 24, 550–559. [Google Scholar] [CrossRef] [Green Version]
- Mantas, A.; Mihranyan, A. Immediate-Release Nifedipine Binary Dry Powder Mixtures with Nanocellulose Featuring Enhanced Solubility and Dissolution Rate. Pharmaceutics 2019, 11, 37. [Google Scholar] [CrossRef] [Green Version]
- Alqurshi, A.; Andrew Chan, K.L.; Royall, P.G. In-Situ Freeze-Drying-Forming Amorphous Solids Directly within Capsules: An Investigation of Dissolution Enhancement for A Poorly Soluble Drug. Sci. Rep. 2017, 7, 2910. [Google Scholar] [CrossRef] [PubMed]
- Ueda, K.; Yamazoe, C.; Yasuda, Y.; Higashi, K.; Kawakami, K.; Moribe, K. Mechanism of Enhanced Nifedipine Dissolution by Polymer-Blended Solid Dispersion through Molecular-Level Characterization. Mol. Pharm. 2018, 15, 4099–4109. [Google Scholar] [CrossRef] [PubMed]
- Taokaew, S.; Ofuchi, M.; Kobayashi, T. Chitin Biomass-Nifedipine Amorphous Solid Dispersion for Enhancement of Hydrophobic Drug Dissolution in Aqueous Media. Sustain. Chem. Pharm. 2020, 17, 100284. [Google Scholar] [CrossRef]
- Zhang, D.; Lee, Y.-C.; Shabani, Z.; Frankenfeld Lamm, C.; Zhu, W.; Li, Y.; Templeton, A. Processing Impact on Performance of Solid Dispersions. Pharmaceutics 2018, 10, 142. [Google Scholar] [CrossRef] [Green Version]
- Tran, P.; Pyo, Y.C.; Kim, D.H.; Lee, S.E.; Kim, J.K.; Park, J.S. Overview of the Manufacturing Methods of Solid Dispersion Technology for Improving the Solubility of Poorly Water-Soluble Drugs and Application to Anticancer Drugs. Pharmaceutics 2019, 11, 132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Duong, T.; Van den Mooter, G. The Role of the Carrier in the Formulation of Pharmaceutical Solid Dispersions. Part I: Crystalline and Semi-Crystalline Carriers. Expert Opin. Drug Deliv. 2016, 13, 1583–1594. [Google Scholar] [CrossRef]
- Chaturvedi, K.; Shah, H.S.; Nahar, K.; Dave, R.; Morris, K.R. Contribution of Crystal Lattice Energy on The Dissolution Behavior of Eutectic Solid Dispersions. ACS Omega 2020, 5, 9690–9701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganesan, P.; Soundararajan, R.; Shanmugam, U.; Ramu, V. Development, Characterization and Solubility Enhancement of Comparative Dissolution Study of Second Generation of Solid Dispersions and Microspheres for Poorly Water Soluble Drug. Asian J. Pharm. Sci. 2015, 10, 433–441. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Tian, L.; Zhu, T.; Mei, J.; Chen, Z.; Yu, D.G. Electrospun Aspirin/Eudragit/Lipid Hybrid Nanofibers for Colon-Targeted Delivery using An Energy-Saving Process. Chem. Res. Chin. Univ. 2021, 37, 443–449. [Google Scholar] [CrossRef]
- Wang, K.; Wen, H.F.; Yu, D.G.; Yang, Y.; Zhang, D.F. Electrosprayed Hydrophilic Nanocomposites Coated with Shellac for Colon-Specific Delayed Drug Delivery. Mater. Des. 2018, 143, 248–255. [Google Scholar] [CrossRef]
- Beig, B.; Niazi, M.B.K.; Jahan, Z.; Kakar, S.J.; Shah, G.A.; Shahid, M.; Zia, M.; Haq, M.U.; Rashid, M.I. Biodegradable Polymer Coated Granular Urea Slows Down n Release Kinetics and Improves Spinach Productivity. Polymers 2020, 12, 2623. [Google Scholar] [CrossRef]
- Azeem, B.; KuShaari, K.; Naqvi, M.; Keong, L.K.; Almesfer, M.K.; Al-Qodah, Z.; Naqvi, S.R.; Elboughdiri, N. Production and Characterization of Controlled Release Urea using Biopolymer and Geopolymer as Coating Materials. Polymers 2020, 12, 400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chokshi, R.J.; Zia, H.; Sandhu, H.K.; Shah, N.H.; Malick, W.A. Improving the Dissolution Rate of Poorly Water Soluble Drug by Solid Dispersion and Solid Solution—Pros and Cons. Drug Deliv. 2007, 14, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Kapsi, S.G.; Ayres, J.W. Processing Factors in Development of Solid Solution Formulation of Itraconazole for Enhancement of Drug Dissolution and Bioavailability. Int. J. Pharm. 2001, 229, 193–203. [Google Scholar] [CrossRef]
- Minhaz, M.; Rahman, M.; Ahsan, M.; Khalipha, A.; Chowdhury, M. Dissolution Enhancement of Poorly Soluble Drug by Solvent Evaporation Method using Hydrophilic Polymer: A Solid Dispersion Technique. Int. J. Pharm. Life Sci. 2012, 1. [Google Scholar] [CrossRef]
- Akbari, J.; Saeedi, M.; Morteza-Semnani, K.; Kelidari, H.R.; Moghanlou, F.S.; Zareh, G.; Rostamkalaei, S.; Sadegh Moghanlou, F.; Zareh, G.; Rostamkalaei, S. The Effect of Tween 20, 60, and 80 on Dissolution Behavior of Sprionolactone in Solid Dispersions Prepared by PEG 6000. Adv. Pharm. Bull. 2015, 5, 435–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gohel, M.C.; Patel, L.D. Processing of Nimesulide-PEG 400-PG-PVP Solid Dispersions: Preparation, Characterization, and In Vitro Dissolution. Drug Dev. Ind. Pharm. 2003, 29, 299–310. [Google Scholar] [CrossRef]
- Carré, A. Polar Interactions at Liquid/Polymer Interfaces. J. Adhes. Sci. Technol. 2007, 21, 961–981. [Google Scholar] [CrossRef]
- Yarce, C.J.; Pineda, D.; Correa, C.E.; Salamanca, C.H. Relationship between Surface Properties and In Vitro Drug Release from A Compressed Matrix Containing an Amphiphilic Polymer Material. Pharmaceuticals 2016, 9, 34. [Google Scholar] [CrossRef] [Green Version]
- Saurí, J.; Suñé-Negre, J.M.; Díaz-Marcos, J.; Vilana, J.; Millán, D.; Ticó, J.R.; Miñarro, M.; Pérez-Lozano, P.; García-Montoya, E. Relationships between Surface Free Energy, Surface Texture Parameters and Controlled Drug Release in Hydrophilic Matrices. Int. J. Pharm. 2015, 478, 328–340. [Google Scholar] [CrossRef]
- Weerapol, Y.; Limmatvapirat, S.; Nunthanid, J.; Konthong, S.; Suttiruengwong, S.; Sriamornsak, P. Development and Characterization of Nifedipine-Amino Methacrylate Copolymer Solid Dispersion Powders with Various Adsorbents. Asian J. Pharm. Sci. 2017, 12, 335–343. [Google Scholar] [CrossRef]
- Genot, C.; Kabri, T.H.; Meynier, A. Stabilization of Omega-3 Oils and Enriched Foods using Emulsifiers. In Food Enrichment with Omega-3 Fatty Acids; Jacobsen, C., Nielsen, N.S., Horn, A.F., Sørensen, A.-D., Eds.; Woodhead Publishing: Sawston, UK, 2013; pp. 150–193. [Google Scholar] [CrossRef]
- Weerapol, Y.; Tubtimsri, S.; Jansakul, C.; Sriamornsak, P. Improved Dissolution of Kaempferia parviflora Extract for Oral Administration by Preparing Solid Dispersion via Solvent Evaporation. Asian J. Pharm. Sci. 2017, 12, 124–133. [Google Scholar] [CrossRef]
- Yang, B.; Wei, C.; Yang, Y.; Wang, Q.; Li, S. Evaluation about Wettability, Water Absorption or Swelling of Excipients through Various Methods and The Correlation between These Parameters and Tablet Disintegration. Drug Dev. Ind. Pharm. 2018, 44, 1417–1425. [Google Scholar] [CrossRef]
- Ponnusamy, T.; Lawson, L.B.; Freytag, L.C.; Blake, D.A.; Ayyala, R.S.; John, V.T. In Vitro Degradation and Release Characteristics of Spin Coated Thin Films of PLGA with A “Breath Figure” Morphology. Biomatter 2012, 2, 77–86. [Google Scholar] [CrossRef] [Green Version]
- Wu, S. Calculation of Interfacial Tension in Polymer Systems. J. Polym. Sci. Part C Polym. Symp. 2007, 34, 19–30. [Google Scholar] [CrossRef]
- Fernández, V.; Khayet, M. Evaluation of the Surface Free Energy of Plant Surfaces: Toward Standardizing the Procedure. Front. Plant Sci. 2015, 6, 510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slavutsky, A.M.; Gamboni, J.E.; Bertuzzi, M.A. Formulation and Characterization of Bilayer Films Based on Brea Gum and Pectin. Braz. J. Food Technol. 2018, 21. [Google Scholar] [CrossRef]
- Weerapol, Y.; Limmatvapirat, S.; Jansakul, C.; Takeuchi, H.; Sriamornsak, P. Enhanced Dissolution and Oral Bioavailability of Nifedipine by Spontaneous Emulsifying Powders: Effect of Solid Carriers and Dietary State. Eur. J. Pharm. Biopharm. 2015, 91, 25–34. [Google Scholar] [CrossRef]
- Szűts, A.; Láng, P.; Ambrus, R.; Kiss, L.; Deli, M.A.; Szabó-Révész, P. Applicability of Sucrose Laurate as Surfactant in Solid Dispersions Prepared by Melt Technology. Int. J. Pharm. 2011, 410, 107–110. [Google Scholar] [CrossRef]
- Linares, V.; Yarce, C.J.; Echeverri, J.D.; Galeano, E.; Salamanca, C.H. Relationship Between Degree of Polymeric Ionisation and Hydrolytic Degradation of Eudragit® E Polymers under Extreme Acid Conditions. Polymers 2019, 11, 1010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikghalb, L.A.; Singh, G.; Singh, G.; Kahkeshan, K.F. Solid Dispersion: Methods and Polymers to Increase the Solubility of Poorly Soluble Drugs. J. Appl. Pharm. Sci. 2012, 2, 170–175. [Google Scholar] [CrossRef]
- Yang, B.; Wu, L.; Ke, J.; Zhou, L.; Chen, M.; Li, S.; Feng, X. Effects of Polymer/Surfactant as Carriers on the Solubility and Dissolution of Fenofibrate Solid Dispersion. AAPS PharmSciTech 2019, 20, 102. [Google Scholar] [CrossRef]
- Dehghana, M.H.G.; Mohammad, J. Improving Dissolution of Meloxicam using Solid Dispersions. Iran. J. Pharm. Res. 2006, 5, 231–238. [Google Scholar] [CrossRef]
- Craig, D.Q.M. The Mechanisms of Drug Release from Solid Dispersions in Water-Soluble Polymers. Int. J. Pharm. 2002, 231, 131–144. [Google Scholar] [CrossRef]
- Yang, B.; Xu, L.; Wang, Q.; Li, S. Modulation of the Wettability of Excipients by Surfactant and its Impacts on the Disintegration and Release of Tablets. Drug Dev. Ind. Pharm. 2016, 42, 1945–1955. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Rudraraju, V.S. Wetting Kinetics: An Alternative Approach towards Understanding the Enhanced Dissolution Rate for Amorphous Solid Dispersion of a Poorly Soluble Drug. AAPS PharmSciTech 2015, 16, 1079–1090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fade, V. Link between Drug Absorption Solubility and Permeability Measurements in Caco-2 cells. J. Pharm. Sci. 1998, 87, 1604–1607. [Google Scholar] [CrossRef]
- Schittny, A.; Huwyler, J.; Puchkov, M. Mechanisms of Increased Bioavailability through Amorphous Solid Dispersions: A Review. Drug Deliv. 2020, 27, 110–127. [Google Scholar] [CrossRef]








| Formulations | Surfactants (%) | NDP (%) | AMCP (%) |
|---|---|---|---|
| SS | 0.00 | 25.00 | 75.00 |
| PS20-01 | 0.10 (polysorbate 20) | 24.95 | 74.95 |
| PS20-03 | 0.30 (polysorbate 20) | 24.85 | 74.85 |
| PS20-06 | 0.60 (polysorbate 20) | 24.70 | 74.70 |
| PS60-01 | 0.10 (polysorbate 60) | 24.95 | 74.95 |
| PS60-03 | 0.30 (polysorbate 60) | 24.85 | 74.85 |
| PS60-06 | 0.60 (polysorbate 60) | 24.70 | 74.70 |
| PS65-01 | 0.10 (polysorbate 65) | 24.95 | 74.95 |
| PS65-03 | 0.30 (polysorbate 65) | 24.85 | 74.85 |
| PS65-06 | 0.60 (polysorbate 65) | 24.70 | 74.70 |
| Formulation | Surface Free Energy (mN/m) | Polar Component (mN/m) | Dispersion Component (mN/m) |
|---|---|---|---|
| PS20-01 | 60.74 ± 0.92 * | 41.48 ± 0.87 * | 19.26 ± 0.45 * |
| PS20-03 | 66.09 ± 0.80 * | 45.2 ± 0.68 * | 20.89 ± 0.64 * |
| PS20-06 | 74.58 ± 0.87 * | 48.4 ± 0.63 * | 26.18 ± 0.52 * |
| PS60-01 | 43.59 ± 0.38 * | 27.23 ± 0.72 * | 16.36 ± 0.49 * |
| PS60-03 | 47.41 ± 0.54 * | 34.03 ± 0.66 * | 13.38 ± 0.51 * |
| PS60-06 | 52.31 ± 0.63 * | 37.25 ± 0.48 * | 15.06 ± 0.50 * |
| PS65-01 | 33.89 ± 0.62 * | 23.9 ± 0.54 * | 9.99 ± 0.65 * |
| PS65-03 | 34.48 ± 0.23 * | 21.13 ± 0.43 * | 13.35 ± 0.62 * |
| PS65-06 | 35.21 ± 0.23 * | 20.03 ± 0.37 * | 15.18 ± 0.32 * |
| SS | 40.43 ± 0.76 | 26.3 ± 0.32 | 14.13 ± 0.30 |
| Glass slide surface | 56.31 ± 0.70 | 31.51 ± 0.69 | 24.8 ± 0.57 |
| Formulations | Q20 (%) | Q120 (%) |
|---|---|---|
| NDP | 14.41 ± 4.51 † | 16.82 ± 1.50 † |
| SS | 51.29 ± 1.52 * | 58.19 ± 2.32 * |
| PS20-01 | 53.37 ± 3.18 *,† | 74.73 ± 5.90 *,† |
| PS20-03 | 75.55 ± 6.33 *,† | 84.26 ± 3.67 *,† |
| PS20-06 | 82.52 ± 0.71 *,† | 95.25 ± 1.47 *,† |
| PS60-01 | 52.07 ± 0.9 *,† | 64.10 ± 6.01 *,† |
| PS60-03 | 52.98 ± 1.03 *,† | 70.34 ± 1.30 *,† |
| PS60-06 | 79.67 ± 2.94 *,† | 89.96 ± 3.04 *,† |
| PS65-01 | 25.69 ± 1.02 *,† | 55.02 ± 3.41 *,† |
| PS65-03 | 27.43 ± 1.79 *,† | 50.87 ± 5.32 *,† |
| PS65-06 | 26.43 ± 1.79 *,† | 43.24 ± 2.76 *,† |
| Formulations | Day 0 (%) | 3 Months (%) | 6 Months (%) | ||
|---|---|---|---|---|---|
| Ambient Condition | Accelerated Condition | Ambient Condition | Accelerated Condition | ||
| SS | 100.03 ± 0.23 | 100.02 ± 0.17 | 100.02 ± 2.37 | 100.01 ± 0.38 | 100.00 ± 3.11 |
| PS20-06 | 100.01 ± 3.27 | 100.03 ± 4.20 | 100.02 ± 1.33 | 100.01 ± 1.27 | 100.02 ± 0.25 |
| PS60-06 | 100.03 ± 4.12 | 100.02 ± 3.25 | 100.01 ± 1.18 | 100.02 ± 3.13 | 100.01 ± 0.13 |
| PS65-06 | 100.03 ± 2.26 | 100.02 ± 1.20 | 100.01 ± 0.33 | 100.02 ± 1.11 | 100.01 ± 0.23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tubtimsri, S.; Weerapol, Y. Improvement in Solubility and Absorption of Nifedipine Using Solid Solution: Correlations between Surface Free Energy and Drug Dissolution. Polymers 2021, 13, 2963. https://doi.org/10.3390/polym13172963
Tubtimsri S, Weerapol Y. Improvement in Solubility and Absorption of Nifedipine Using Solid Solution: Correlations between Surface Free Energy and Drug Dissolution. Polymers. 2021; 13(17):2963. https://doi.org/10.3390/polym13172963
Chicago/Turabian StyleTubtimsri, Sukannika, and Yotsanan Weerapol. 2021. "Improvement in Solubility and Absorption of Nifedipine Using Solid Solution: Correlations between Surface Free Energy and Drug Dissolution" Polymers 13, no. 17: 2963. https://doi.org/10.3390/polym13172963