Biomaterial-Assisted Anastomotic Healing: Serosal Adhesion of Pectin Films
Abstract
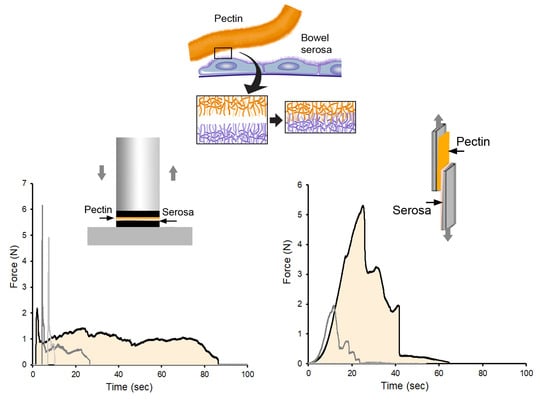
:1. Introduction
2. Methods
2.1. Animals
2.2. Lectin Histochemistry
2.3. Lectins
2.4. Pectin
2.5. Pectin Dissolution in Water
2.6. Nanocellulose Fibers (NCF)
2.7. Pressure-Sensitive Adhesive (PSA)
2.8. Surgical Sealants
2.9. Adhesion Testing: Tensile Adhesion Strength
2.10. Adhesion Testing: Shear Resistance
2.11. Transmission Electron Microscopy
2.12. Static Pressure Simulacrum
2.13. Luminal Pressure Simulation
2.14. Statistical Analysis
3. Results
3.1. Pectin and MPS Adherends
3.2. Tensile Adhesion Strength
3.3. Shear Resistance
3.4. Luminal Pressure Simulation
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Favoriti, P.; Carbone, G.; Greco, M.; Pirozzi, F.; Pirozzi, R.E.; Corcione, F. Worldwide burden of colorectal cancer: A review. Updates Surg. 2016, 68, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Mattiuzzi, C.; Sanchis-Gomar, F.; Lippi, G. Concise update on colorectal cancer epidemiology. Ann. Transl. Med. 2019, 7, 609. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global Cancer Incidence and Mortality Rates and Trends--An Update. Cancer Epidemiol. Biomarkers Prev. 2016, 25, 16–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mármol, I.; Sánchez-de-Diego, C.; Pradilla Dieste, A.; Cerrada, E.; Rodriguez Yoldi, M.J. Colorectal Carcinoma: A General Overview and Future Perspectives in Colorectal Cancer. Int. J. Mol. Sci. 2017, 18, 197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kakeji, Y.; Oshikiri, T.; Takiguchi, G.; Kanaji, S.; Matsuda, T.; Nakamura, T.; Suzuki, S. Multimodality approaches to control esophageal cancer: Development of chemoradiotherapy, chemotherapy, and immunotherapy. Esophagus 2021, 18, 25–32. [Google Scholar] [CrossRef]
- Kaiser, A.M. State of the art in gastrointestinal surgery 100 years ago: Operations in the gastrointestinal tract in general (Chapter XII) and resection of bowel carcinoma (Chapter XIV) from the Textbook of Special Surgery (1897) by Eduard Albert (1841–1900). World J. Surg. 2002, 26, 1525–1530. [Google Scholar] [CrossRef] [PubMed]
- Boschung, U. Milestones in the history of intestinal anastomosis. Swiss Surg. Schweiz. Chir. Chir. Suisse Chir. Svizz. 2003, 9, 99–104. [Google Scholar]
- Dietz, U.A.; Debus, E.S. Intestinal anastomoses prior to 1882; a legacy of ingenuity, persistence, and research form a foundation for modern gastrointestinal surgery. World J. Surg. 2005, 29, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.S.; Margolin, D.A. Management of Colorectal Anastomotic Leak. Cllin. Colon Rectal Surg. 2016, 29, 138–144. [Google Scholar] [CrossRef] [Green Version]
- van Praagh, J.B.; de Goffau, M.C.; Bakker, I.S.; Harmsen, H.J.M.; Olinga, P.; Havenga, K. Intestinal microbiota and anastomotic leakage of stapled colorectal anastomoses: A pilot study. Surg. Endosc. 2016, 30, 2259–2265. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Huh, J.W.; Park, Y.A.; Cho, Y.B.; Yun, S.H.; Kim, H.C.; Lee, W.Y. Risk Factors of Anastomotic Leakage and Long-Term Survival After Colorectal Surgery. Medicine 2016, 95, 1–10. [Google Scholar] [CrossRef] [PubMed]
- McDermott, F.D.; Heeney, A.; Kelly, M.E.; Steele, R.J.; Carlson, G.L.; Winter, D.C. Systematic review of preoperative, intraoperative and postoperative risk factors for colorectal anastomotic leaks. Br. J. Surg. 2015, 102, 462–479. [Google Scholar] [CrossRef] [PubMed]
- Hyman, N.; Manchester, T.L.; Osler, T.; Burns, B.; Cataldo, P.A. Anastomotic leaks after intestinal anastomosis—It’s later than you think. Ann. Surg. 2007, 245, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Phillips, B.R. Reducing gastrointestinal anastomotic leak rates: Review of challenges and solutions. Open Access Surg. 2016, 9, 5–14. [Google Scholar] [CrossRef] [Green Version]
- Hinz, B. The role of myofibroblasts in wound healing. Curr. Res. Transl. Med. 2016, 64, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Nordentoft, T.; Pommergaard, H.-C.; Rosenberg, J.; Achiam, M.P. Fibrin Glue Does Not Improve Healing of Gastrointestinal Anastomoses: A Systematic Review. Eur. Surg. Res. 2015, 54, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Gagner, M.; Kemmeter, P. Comparison of laparoscopic sleeve gastrectomy leak rates in five staple-line reinforcement options: A systematic review. Surg. Endosc. 2020, 34, 396–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Placer, C.; Enríquez-Navascués, J.M.; Elorza, G.; Timoteo, A.; Mugica, J.A.; Borda, N.; Saralegui, Y.; Elósegui, J.L. Preventing complications in colorectal anastomosis: Results of a randomized controlled trial using bioabsorbable staple line reinforcement for circular stapler. Dis. Colon Rectum 2014, 57, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.J.; Kim, J.; Kwak, J.; Kim, S.H. Can trans-anal reinforcing sutures after double stapling in lower anterior resection reduce the need for a temporary diverting ostomy? World J. Gastroenterol. 2013, 19, 5309–5313. [Google Scholar] [CrossRef]
- Kim, I.Y.; Kim, B.R.; Kim, Y.W. Applying reinforcing sutures to stapled colorectal anastomosis after low anterior resection for rectal cancer. Eur. J. Surg. Oncol. 2015, 41, 808–809. [Google Scholar] [CrossRef]
- Mohan, H.M.; Winter, D.C. Autobuttressing of colorectal anastomoses using a mesenteric flap. Updates Surg. 2013, 65, 333–335. [Google Scholar] [CrossRef]
- Yuan, Y.; Zeng, X.; Hu, Y.; Xie, T.; Zhao, Y. Omentoplasty for oesophagogastrostomy after oesophagectomy. Cochrane Database Syst. Rev. 2014, 10, Cd008446. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.; Naiken, S.; Christou, N.; Liot, E.; Toso, C.; Buchs, N.C.; Ris, F. Reducing anastomotic leak in colorectal surgery: The old dogmas and the new challenges. World J. Gastroenterol. 2019, 25, 5017–5025. [Google Scholar] [CrossRef]
- Wagner, W.L.; Zheng, Y.; Pierce, A.; Ackermann, M.; Horstmann, H.; Kuner, T.; Ronchi, P.; Schwab, Y.; Konietzke, P.; Wunnemann, F.; et al. Mesopolysaccharides: The extracellular surface layer of visceral organs. PLoS ONE 2020, 15, e0238798. [Google Scholar] [CrossRef]
- Scheller, H.V.; Jensen, J.K.; Sorensen, S.O.; Harholt, J.; Geshi, N. Biosynthesis of pectin. Physiol. Plant 2007, 129, 283–295. [Google Scholar] [CrossRef]
- Monsoor, M.A.; Kalapathy, U.; Proctor, A. Determination of polygalacturonic acid content in pectin extracts by diffuse reflectance Fourier transform infrared spectroscopy. Food Chem. 2001, 74, 233–238. [Google Scholar] [CrossRef]
- Nunes, C.; Silva, L.; Fernandes, A.P.; Guine, R.P.F.; Domingues, M.R.M.; Coimbra, M.A. Occurrence of cellobiose residues directly linked to galacturonic acid in pectic polysaccharides. Carbohydr. Polym. 2012, 87, 620–626. [Google Scholar] [CrossRef]
- Servais, A.B.; Kienzle, A.; Valenzuela, C.D.; Ysasi, A.B.; Wagner, W.L.; Tsuda, A.; Ackermann, M.; Mentzer, S.J. Structural heteropolysaccharide adhesion to the glycocalyx of visceral mesothelium. Tissue Eng. Part A 2018, 24, 199–206. [Google Scholar] [CrossRef]
- Servais, A.B.; Kienzle, A.; Ysasi, A.B.; Valenzuela, C.D.; Wagner, W.L.; Tsuda, A.; Ackermann, M.; Mentzer, S.J. Structural heteropolysaccharides as air-tight sealants of the human pleura. J. Biol. Mat. Res. 2018, 107, 799–806. [Google Scholar] [CrossRef]
- Pierce, A.; Zheng, Y.; Wagner, W.L.; Scheller, H.V.; Mohnen, D.; Tsuda, A.; Ackermann, M.; Mentzer, S.J. Visualizing pectin polymer-polymer entanglement produced by interfacial water movement. Carbohydr. Polym. 2020, 246, 116618. [Google Scholar] [CrossRef] [PubMed]
- Chen, C. The art of bowel anastomosis. Scand. J. Surg. 2012, 101, 238–240. [Google Scholar] [CrossRef]
- Pierce, A.; Zheng, Y.; Wagner, W.L.; Scheller, H.V.; Mohnen, D.; Tsuda, A.; Ackermann, M.; Mentzer, S.J. Pectin biopolymer mechanics and microstructure associated with polysaccharide phase transitions. J. Biol. Mat. Res. Part A 2020, 108, 246–253. [Google Scholar] [CrossRef]
- Gibney, B.; Lee, G.S.; Houdek, J.; Lin, M.; Chamoto, K.; Konerding, M.A.; Tsuda, A.; Mentzer, S.J. Dynamic determination of oxygenation and lung compliance in murine pneumonectomy. Exp. Lung Res. 2011, 37, 301–309. [Google Scholar] [CrossRef] [Green Version]
- Reeke, G.N., Jr.; Becker, J.W.; Cunningham, B.A.; Gunther, G.R.; Wang, J.L.; Edelman, G.M. Relationships between the structure and activities of concanavalin A. Ann. N. Y. Acad. Sci. 1974, 234, 369–382. [Google Scholar] [CrossRef]
- Matsumoto, I.; Jimbo, A.; Mizuno, Y.; Seno, N.; Jeanloz, R.W. Purification and characterization of potato lectin. J. Biol. Chem. 1983, 258, 2886–2891. [Google Scholar] [CrossRef]
- Biswal, A.K.; Tan, L.; Atmodjo, M.A.; DeMartini, J.; Gelineo-Albersheim, I.; Hunt, K.; Black, I.M.; Mohanty, S.S.; Ryno, D.; Wyman, C.E.; et al. Comparison of four glycosyl residue composition methods for effectiveness in detecting sugars from cell walls of dicot and grass tissues. Biotechnol. Biofuels 2017, 10, 1–18. [Google Scholar] [CrossRef]
- Zheng, Y.; Pierce, A.; Wagner, W.L.; Scheller, H.V.; Mohnen, D.; Ackermann, M.; Mentzer, S.J. Water-dependent blending of pectin films: The mechanics of conjoined biopolymers. Molecules 2020, 30, 2108. [Google Scholar] [CrossRef] [PubMed]
- Luft, J.H. Fine structures of capillary and endocapillary layer as revealed by ruthenium red. Fed. Proc. 1966, 25, 1773–1783. [Google Scholar] [PubMed]
- Chevalier, L.; Selim, J.; Genty, D.; Baste, J.M.; Piton, N.; Boukhalfa, I.; Hamzaoui, M.; Pareige, P.; Richard, V. Electron microscopy approach for the visualization of the epithelial and endothelial glycocalyx. Morphologie 2017, 101, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Scott, S.M.; Knowles, C.H.; Wang, D.; Yazaki, E.; Picon, L.; Wingate, D.L.; Lindberg, G. The nocturnal jejunal migrating motor complex: Defining normal ranges by study of 51 healthy adult volunteers and meta-analysis. Neurogastroenterol. Motil. 2006, 18, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Grivel, M.L.; Ruckebusch, Y. The propagation of segmental contractions along the small intestine. J. Physiol. 1972, 227, 611–625. [Google Scholar] [CrossRef] [Green Version]
- Otterson, M.F.; Sarr, M.G. Normal physiology of small intestinal motility. Surg. Clin. North Am. 1993, 73, 1173–1192. [Google Scholar] [CrossRef]
- Hennig, G.W.; Costa, M.; Chen, B.N.; Brookes, S.J.H. Quantitative analysis of peristalsis in the guinea-pig small intestine using spatio-temporal maps. J. Physiol. 1999, 517, 575–590. [Google Scholar] [CrossRef]
- Granger, D.N.; Barrowman, J.A. Microcirculation of the alimentary-tract I. Physiology of transcapillary fluid and solute exchange. Gastroenterology 1983, 84, 846–868. [Google Scholar]
- Brodribb, A.J.M.; Condon, R.E.; Cowles, V.; Decosse, J.J. Effect of dietary fiber on intraluminal pressure and myoelectrical activity of left colon in monkeys. Gastroenterology 1979, 77, 70–74. [Google Scholar] [CrossRef]
- Basson, M.D.; Yu, C.F.; Herden-Kirchoff, O.; Ellermeier, M.; Sanders, M.A.; Merrell, R.C.; Sumpio, B.E. Effects of increased ambient pressure on colon cancer cell adhesion. J. Cell. Biochem. 2000, 78, 47–61. [Google Scholar] [CrossRef]
- Kellow, J.E.; Phillips, S.F. Small-bowel dysmotility correlates with symptoms in irritable-bowel-syndrome. Gastroenterology 1986, 90, 1488. [Google Scholar]
- Alizadeh, H.; Weems, W.A.; Castro, G.A. Long-term influence of enteric infection on jejunal propulsion in guinea-pigs. Gastroenterology 1989, 97, 1461–1468. [Google Scholar] [CrossRef]
- Gurunluoglu, R.; Gurunluoglu, A.; Piza-Katzer, H. Review of the “Chirurgia” of Giovanni de Vigo: Estimate of his position in the history of surgery. World J. Surg. 2003, 27, 616–623. [Google Scholar] [CrossRef]
- Nockemann, P.E. The invagination operation of Ramdohr and its importance for the development of intestinal suture. Chirurg 2000, 71, 1296–1300. [Google Scholar] [CrossRef]
- Senn, N. Enterorrhaphy; its history, technique and present status. JAMA 1893, 21, 215–235. [Google Scholar]
- Andree, B.; Bar, A.; Haverich, A.; Hilfiker, A. Small Intestinal Submucosa Segments as Matrix for Tissue Engineering: Review. Tissue Eng. Part B 2013, 19, 279–291. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Y.; Pierce, A.F.; Wagner, W.L.; Khalil, H.A.; Chen, Z.; Funaya, C.; Ackermann, M.; Mentzer, S.J. Biomaterial-Assisted Anastomotic Healing: Serosal Adhesion of Pectin Films. Polymers 2021, 13, 2811. https://doi.org/10.3390/polym13162811
Zheng Y, Pierce AF, Wagner WL, Khalil HA, Chen Z, Funaya C, Ackermann M, Mentzer SJ. Biomaterial-Assisted Anastomotic Healing: Serosal Adhesion of Pectin Films. Polymers. 2021; 13(16):2811. https://doi.org/10.3390/polym13162811
Chicago/Turabian StyleZheng, Yifan, Aidan F. Pierce, Willi L. Wagner, Hassan A. Khalil, Zi Chen, Charlotta Funaya, Maximilian Ackermann, and Steven J. Mentzer. 2021. "Biomaterial-Assisted Anastomotic Healing: Serosal Adhesion of Pectin Films" Polymers 13, no. 16: 2811. https://doi.org/10.3390/polym13162811