Monomer Conversion, Dimensional Stability, Biaxial Flexural Strength, Ion Release, and Cytotoxicity of Resin-Modified Glass Ionomer Cements Containing Methacrylate-Functionalized Polyacids and Spherical Pre-Reacted Glass Fillers
Abstract
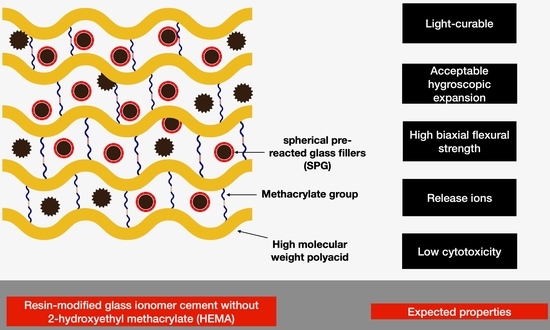
:1. Introduction
2. Materials and Methods
2.1. Material Preparation
2.2. Degree of Monomer Conversion
2.3. Biaxial Flexural Strength (BFS) and Biaxial Flexural Modulus (BFM)
2.4. Dimensional Stability (Mass and Volume Changes)
2.5. Ion. Release
2.5.1. Fluoride Release
2.5.2. Aluminum, Calcium, Strontium, and Sodium Ion Release
2.6. Cytotoxicity Test
2.6.1. Direct Contact Cytotoxicity Test
2.6.2. Indirect Contact Cytotoxicity Test
2.7. Statistical Analysis
3. Results
3.1. Degree of Monomer Conversion
3.2. Dimensional Stability (Mass and Volume Changes)
3.3. Biaxial Flexural Strength (BFS) and Biaxial Flexural Modulus (BFM)
3.4. Ion. Release
3.5. Cytotoxicity Test
4. Discussion
4.1. Degree of Monomer Conversion
4.2. Dimensional Stability (Mass and Volume Changes)
4.3. Biaxial Flexural Strength
4.4. Ion. Release
4.5. Cytotoxicity Test
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GBD Oral Disorders Collaborators. Global, Regional, and National Levels and Trends in Burden of Oral Conditions from 1990 to 2017: A Systematic Analysis for the Global Burden of Disease 2017 Study. J. Dent. Res. 2020, 99, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Schwendicke, F.; Splieth, C.; Breschi, L.; Banerjee, A.; Fontana, M.; Paris, S.; Burrow, M.F.; Crombie, F.; Page, L.F.; Gaton-Hernandez, P.; et al. When to intervene in the caries process? An expert Delphi consensus statement. Clin. Oral Investig. 2019, 23, 3691–3703. [Google Scholar] [CrossRef]
- Schwendicke, F. Less Is More? The Long-Term Health and Cost Consequences Resulting from Minimal Invasive Caries Management. Dent. Clin. North. Am. 2019, 63, 737–749. [Google Scholar] [CrossRef]
- Schwendicke, F. Contemporary concepts in carious tissue removal: A review. J. Esthet Restor. Dent. 2017, 29, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Mittal, S.; Tewari, S. Effect of Different Liners on Pulpal Outcome after Partial Caries Removal: A Preliminary 12 Months Randomised Controlled Trial. Caries Res. 2019, 53, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Chai, B.; Tay, B.; Chow, C.; Fuss, J.; Krishnan, U. Treatment preferences for deep caries lesions among Australian dentists. Aust. Dent. J. 2020, 65, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Blum, I.R.; Wilson, N.H.F. Consequences of no more linings under composite restorations. Br. Dent. J. 2019, 226, 749–752. [Google Scholar] [CrossRef]
- Ranjbar Omrani, L.; Moradi, Z.; Abbasi, M.; Kharazifard, M.J.; Tabatabaei, S.N. Evaluation of Compressive Strength of Several Pulp Capping Materials. J. Dent. 2021, 22, 41–47. [Google Scholar] [CrossRef]
- de Mello Torres, A.C.; Gomes, A.P.M.; Kubo, C.H.; Torres, C.R.G. Protection of the Dentin-Pulp Complex. In Modern Operative Dentistry; Torres, C.R.G., Ed.; Textbooks in Contemporary Dentistry; Springer International Publishing: Cham, Switzerland, 2020; pp. 289–333. [Google Scholar]
- Bjorndal, L.; Simon, S.; Tomson, P.L.; Duncan, H.F. Management of deep caries and the exposed pulp. Int. Endod. J. 2019, 52, 949–973. [Google Scholar] [CrossRef]
- Sidhu, S.K.; Nicholson, J.W. A review of glass-Ionomer cements for clinical dentistry. J. Funct Biomater. 2016, 7, 16. [Google Scholar] [CrossRef]
- Zanchi, C.H.; Munchow, E.A.; Ogliari, F.A.; de Carvalho, R.V.; Chersoni, S.; Prati, C.; Demarco, F.F.; Piva, E. A new approach in self-etching adhesive formulations: Replacing HEMA for surfactant dimethacrylate monomers. J. Biomed. Mater. Res. B Appl. Biomater. 2011, 99, 51–57. [Google Scholar] [CrossRef]
- Ferracane, J.L. Hygroscopic and hydrolytic effects in dental polymer networks. Dent. Mater. 2006, 22, 211–222. [Google Scholar] [CrossRef]
- Bociong, K.; Szczesio, A.; Sokolowski, K.; Domarecka, M.; Sokolowski, J.; Krasowski, M.; Lukomska-Szymanska, M. The Influence of Water Sorption of Dental Light-Cured Composites on Shrinkage Stress. Materials 2017, 10, 1142. [Google Scholar] [CrossRef]
- Meriwether, L.A.; Blen, B.J.; Benson, J.H.; Hatch, R.H.; Tantbirojn, D.; Versluis, A. Shrinkage stress compensation in composite-restored teeth: Relaxation or hygroscopic expansion? Dent. Mater. 2013, 29, 573–579. [Google Scholar] [CrossRef]
- Williams, D.W.; Wu, H.; Oh, J.E.; Fakhar, C.; Kang, M.K.; Shin, K.H.; Park, N.H.; Kim, R.H. 2-Hydroxyethyl methacrylate inhibits migration of dental pulp stem cells. J. Endod. 2013, 39, 1156–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jochums, A.; Volk, J.; Perduns, R.; Plum, M.; Schertl, P.; Bakopoulou, A.; Geurtsen, W. Influence of 2-hydroxyethyl methacrylate (HEMA) exposure on angiogenic differentiation of dental pulp stem cells (DPSCs). Dent. Mater. 2021, 37, 534–546. [Google Scholar] [CrossRef] [PubMed]
- Baldion, P.A.; Velandia-Romero, M.L.; Castellanos, J.E. Dental resin monomers induce early and potent oxidative damage on human odontoblast-like cells. Chem. Biol. Interact. 2021, 333, 109336. [Google Scholar] [CrossRef] [PubMed]
- Massaro, H.; Zambelli, L.F.A.; Britto, A.A.; Vieira, R.P.; Ligeiro-de-Oliveira, A.P.; Andia, D.C.; Oliveira, M.T.; Lima, A.F. Solvent and HEMA Increase Adhesive Toxicity and Cytokine Release from Dental Pulp Cells. Materials 2019, 12, 2750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, R.S.; Lee, M.S.; Hu, Y.J.; Hu, C.Y.; Tseng, W.Y. The effects of low-dose 2-hydroxyethyl methacrylate on apoptosis and survival in human dental pulp cells. J. Formos. Med. Assoc. 2021, 120, 1332–1339. [Google Scholar] [CrossRef]
- Thepveera, W.; Potiprapanpong, W.; Toneluck, A.; Channasanon, S.; Khamsuk, C.; Monmaturapoj, N.; Tanodekaew, S.; Panpisut, P. Rheological Properties, Surface Microhardness, and Dentin Shear Bond Strength of Resin-Modified Glass Ionomer Cements Containing Methacrylate-Functionalized Polyacids and Spherical Pre-Reacted Glass Fillers. J. Funct. Biomater. 2021, 12, 42. [Google Scholar] [CrossRef]
- Monmaturapoj, N.; Soodsawang, W.; Tanodekaew, S. Enhancement effect of pre-reacted glass on strength of glass-ionomer cement. Dent. Mater. J. 2012, 31, 125–130. [Google Scholar] [CrossRef] [Green Version]
- Zafar, M.S. Effects of surface pre-reacted glass particles on fluoride release of dental restorative materials. World Appl. Sci. J. 2013, 28, 457–462. [Google Scholar]
- Channasanon, S.; Soodsawang, W.; Monmaturapoj, N.; Tanodekaew, S. Improving flexural strength of resin modified glass-ionomer cement by poly (alkenoic acid) modifications. Songklanakarin J. Sci. Technol. 2013, 35, 429–436. [Google Scholar]
- Panpisut, P.; Monmaturapoj, N.; Srion, A.; Toneluck, A.; Phantumvanit, P. Physical properties of glass ionomer cement containing pre-reacted spherical glass fillers. Braz. Dent. J. 2020, 31, 445–452. [Google Scholar] [CrossRef]
- Panpisut, P.; Monmaturapoj, N.; Srion, A.; Angkananuwat, C.; Krajangta, N.; Panthumvanit, P. The effect of powder to liquid ratio on physical properties and fluoride release of glass ionomer cements containing pre-reacted spherical glass fillers. Dent. Mater. J. 2020, 39, 563–570. [Google Scholar] [CrossRef] [Green Version]
- Panpisut, P.; Suppapatpong, T.; Rattanapan, A.; Wongwarawut, P. Monomer conversion, biaxial flexural strength, apatite forming ability of experimental dual-cured and self-adhesive dental composites containing calcium phosphate and nisin. Dent. Mater. J. 2021, 40, 399–406. [Google Scholar] [CrossRef]
- Delgado, A.H.S.; Young, A.M. Methacrylate peak determination and selection recommendations using ATR-FTIR to investigate polymerisation of dental methacrylate mixtures. PLoS ONE 2021, 16, e0252999. [Google Scholar] [CrossRef]
- British Standard. Dentistry-Water-Based Cements. Part 2: Resin-Modified Cements (ISO 9917-2:2017); British Standard: London, UK, 2017. [Google Scholar]
- Panpisut, P.; Toneluck, A. Monomer conversion, dimensional stability, biaxial flexural strength, and fluoride release of resin-based restorative material containing alkaline fillers. Dent. Mater. J. 2020, 39, 608–615. [Google Scholar] [CrossRef] [Green Version]
- Panpisut, P.; Liaqat, S.; Zacharaki, E.; Xia, W.; Petridis, H.; Young, A.M. Dental composites with calcium/strontium phosphates and polylysine. PLoS ONE 2016, 11, e0164653. [Google Scholar] [CrossRef]
- Kangwankai, K.; Sani, S.; Panpisut, P.; Xia, W.; Ashley, P.; Petridis, H.; Young, A.M. Monomer conversion, dimensional stability, strength, modulus, surface apatite precipitation and wear of novel, reactive calcium phosphate and polylysine-containing dental composites. PLoS ONE 2017, 12, e0187757. [Google Scholar] [CrossRef] [Green Version]
- Pagano, S.; Lombardo, G.; Balloni, S.; Bodo, M.; Cianetti, S.; Barbati, A.; Montaseri, A.; Marinucci, L. Cytotoxicity of universal dental adhesive systems: Assessment in vitro assays on human gingival fibroblasts. Toxicol. In Vitro 2019, 60, 252–260. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Moldovan, M.; Balazsi, R.; Soanca, A.; Roman, A.; Sarosi, C.; Prodan, D.; Vlassa, M.; Cojocaru, I.; Saceleanu, V.; Cristescu, I. Evaluation of the Degree of Conversion, Residual Monomers and Mechanical Properties of Some Light-Cured Dental Resin Composites. Materials 2019, 12, 2109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goncalves, F.; Campos, L.M.P.; Rodrigues-Junior, E.C.; Costa, F.V.; Marques, P.A.; Francci, C.E.; Braga, R.R.; Boaro, L.C.C. A comparative study of bulk-fill composites: Degree of conversion, post-gel shrinkage and cytotoxicity. Braz. Oral Res. 2018, 32, e17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguiar, T.R.; de Oliveira, M.; Arrais, C.A.; Ambrosano, G.M.; Rueggeberg, F.; Giannini, M. The effect of photopolymerization on the degree of conversion, polymerization kinetic, biaxial flexure strength, and modulus of self-adhesive resin cements. J. Prosthet. Dent. 2015, 113, 128–134. [Google Scholar] [CrossRef]
- Sideridou, I.; Tserki, V.; Papanastasiou, G. Effect of chemical structure on degree of conversion in light-cured dimethacrylate-based dental resins. Biomaterials 2002, 23, 1819–1829. [Google Scholar] [CrossRef]
- Achilias, D.; Siafaka, P. Polymerization Kinetics of Poly(2-Hydroxyethyl Methacrylate) Hydrogels and Nanocomposite Materials. Processes 2017, 5, 21. [Google Scholar] [CrossRef] [Green Version]
- Habib, E.; Wang, R.; Zhu, X.X. Correlation of resin viscosity and monomer conversion to filler particle size in dental composites. Dent. Mater. 2018, 34, 1501–1508. [Google Scholar] [CrossRef]
- Calixto, L.R.; Tonetto, M.R.; Pinto, S.C.; Barros, E.D.; Borges, A.H.; Lima, F.V.; de Andrade, M.F.; Bandeca, M.C. Degree of conversion and hardness of two different systems of the Vitrebond glass ionomer cement light cured with blue LED. J. Contemp. Dent. Pract. 2013, 14, 244–249. [Google Scholar] [CrossRef]
- Ribeiro, B.C.; Boaventura, J.M.; Brito-Goncalves, J.; Rastelli, A.N.; Bagnato, V.S.; Saad, J.R. Degree of conversion of nanofilled and microhybrid composite resins photo-activated by different generations of LEDs. J. Appl. Oral Sci. 2012, 20, 212–217. [Google Scholar] [CrossRef]
- Randolph, L.D.; Palin, W.M.; Bebelman, S.; Devaux, J.; Gallez, B.; Leloup, G.; Leprince, J.G. Ultra-fast light-curing resin composite with increased conversion and reduced monomer elution. Dent. Mater. 2014, 30, 594–604. [Google Scholar] [CrossRef]
- Yoshihara, K.; Nagaoka, N.; Okihara, T.; Irie, M.; Matsukawa, A.; Pedano, M.S.; Maruo, Y.; Yoshida, Y.; Van Meerbeek, B. Development of self-adhesive pulp-capping agents containing a novel hydrophilic and highly polymerizable acrylamide monomer. J. Mater. Chem. B 2020, 8, 5320–5329. [Google Scholar] [CrossRef]
- Czarnecka, B.; Nicholson, J.W. Ion release by resin-modified glass-ionomer cements into water and lactic acid solutions. J. Dent. 2006, 34, 539–543. [Google Scholar] [CrossRef]
- Sokolowski, K.; Szczesio-Wlodarczyk, A.; Bociong, K.; Krasowski, M.; Fronczek-Wojciechowska, M.; Domarecka, M.; Sokolowski, J.; Lukomska-Szymanska, M. Contraction and Hydroscopic Expansion Stress of Dental Ion-Releasing Polymeric Materials. Polymers 2018, 10, 1093. [Google Scholar] [CrossRef] [Green Version]
- Rad, I.Y.; Lewis, S.; Barros, M.D.; Kipper, M.; Stansbury, J.W. Suppression of hydrolytic degradation in labile polymer networks via integrated styrenic nanogels. Dent. Mater. 2021, 37, 1295–1306. [Google Scholar] [CrossRef]
- Aljabo, A.; Xia, W.; Liaqat, S.; Khan, M.; Knowles, J.; Ashley, P.; Young, A. Conversion, shrinkage, water sorption, flexural strength and modulus of re-mineralizing dental composites. Dent. Mater. 2015, 31, 1279–1289. [Google Scholar] [CrossRef] [Green Version]
- Kumar, N.; Ghani, F.; Fareed, M.A.; Riaz, S.; Khurshid, Z.; Zafar, M.S. Bi-axial flexural strength of resin based dental composites—Influence and reliability of the testing method configuration. Mater. Technol. 2021, 1–7. [Google Scholar] [CrossRef]
- Ilie, N.; Hilton, T.J.; Heintze, S.D.; Hickel, R.; Watts, D.C.; Silikas, N.; Stansbury, J.W.; Cadenaro, M.; Ferracane, J.L. Academy of Dental Materials guidance-Resin composites: Part I-Mechanical properties. Dent. Mater. 2017, 33, 880–894. [Google Scholar] [CrossRef]
- Beriat, N.C.; Nalbant, D. Water Absorption and HEMA Release of Resin-Modified Glass-Ionomers. Eur. J. Dent. 2009, 3, 267–272. [Google Scholar] [CrossRef] [Green Version]
- Elbishari, H.; Silikas, N.; Satterthwaite, J. Filler size of resin-composites, percentage of voids and fracture toughness: Is there a correlation? Dent. Mater. J. 2012, 31, 523–527. [Google Scholar] [CrossRef]
- Drummond, J.L. Degradation, fatigue, and failure of resin dental composite materials. J. Dent. Res. 2008, 87, 710–719. [Google Scholar] [CrossRef] [Green Version]
- Pedano, M.S.; Yoshihara, K.; Li, X.; Camargo, B.; Van Landuyt, K.; Van Meerbeek, B. Experimental resin-modified calcium-silicate cement containing N-(2-hydroxyethyl) acrylamide monomer for pulp tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 126, 112105. [Google Scholar] [CrossRef] [PubMed]
- Torres, F.F.E.; Guerreiro-Tanomaru, J.M.; Bosso-Martelo, R.; Chavez-Andrade, G.M.; Tanomaru Filho, M. Solubility, porosity and fluid uptake of calcium silicate-based cements. J. Appl. Oral Sci. 2018, 26, e20170465. [Google Scholar] [CrossRef]
- Nicholson, J.W.; Coleman, N.J.; Sidhu, S.K. Kinetics of ion release from a conventional glass-ionomer cement. J. Mater. Sci. Mater. Med. 2021, 32, 30. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, S.K. Glass-ionomer cement restorative materials: A sticky subject? Aust. Dent. J. 2011, 56, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Pires, P.M.; Neves, A.A.; Makeeva, I.M.; Schwendicke, F.; Faus-Matoses, V.; Yoshihara, K.; Banerjee, A.; Sauro, S. Contemporary restorative ion-releasing materials: Current status, interfacial properties and operative approaches. Br. Dent. J. 2020, 229, 450–458. [Google Scholar] [CrossRef]
- Bussola Tovani, C.; Gloter, A.; Azais, T.; Selmane, M.; Ramos, A.P.; Nassif, N. Formation of stable strontium-rich amorphous calcium phosphate: Possible effects on bone mineral. Acta Biomater. 2019, 92, 315–324. [Google Scholar] [CrossRef]
- Lippert, F.; Hara, A.T. Strontium and caries: A long and complicated relationship. Caries Res. 2013, 47, 34–49. [Google Scholar] [CrossRef]
- Gallorini, M.; Cataldi, A.; di Giacomo, V. HEMA-induced cytotoxicity: Oxidative stress, genotoxicity and apoptosis. Int. Endod. J. 2014, 47, 813–818. [Google Scholar] [CrossRef]
- British Standard. 10993–5: 2009 Biological Evaluation of Medical Devices. Part 5: Tests for In Vitro Cytotoxicity; British Standard: London, UK, 2009. [Google Scholar]
- Benetti, F.; Gomes-Filho, J.E.; de Araujo Lopes, J.M.; Barbosa, J.G.; Jacinto, R.C.; Cintra, L.T.A. In vivo biocompatibility and biomineralization of calcium silicate cements. Eur. J. Oral Sci. 2018, 126, 326–333. [Google Scholar] [CrossRef]
- Cintra, L.T.A.; Benetti, F.; de Azevedo Queiroz, I.O.; de Araujo Lopes, J.M.; Penha de Oliveira, S.H.; Sivieri Araujo, G.; Gomes-Filho, J.E. Cytotoxicity, Biocompatibility, and Biomineralization of the New High-plasticity MTA Material. J. Endod. 2017, 43, 774–778. [Google Scholar] [CrossRef] [Green Version]
- Koutroulis, A.; Kuehne, S.A.; Cooper, P.R.; Camilleri, J. The role of calcium ion release on biocompatibility and antimicrobial properties of hydraulic cements. Sci. Rep. 2019, 9, 19019. [Google Scholar] [CrossRef] [Green Version]
- Monticelli, F.; Osorio, R.; Pisani-Proenca, J.; Toledano, M. Resistance to degradation of resin-dentin bonds using a one-step HEMA-free adhesive. J. Dent. 2007, 35, 181–186. [Google Scholar] [CrossRef]
- Szczesio-Wlodarczyk, A.; Sokolowski, J.; Kleczewska, J.; Bociong, K. Ageing of Dental Composites Based on Methacrylate Resins-A Critical Review of the Causes and Method of Assessment. Polymers 2020, 12, 882. [Google Scholar] [CrossRef] [PubMed]
- Skallevold, H.E.; Rokaya, D.; Khurshid, Z.; Zafar, M.S. Bioactive Glass Applications in Dentistry. Int. J. Mol. Sci. 2019, 20, 5960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walters, N.J.; Xia, W.; Salih, V.; Ashley, P.F.; Young, A.M. Poly(propylene glycol) and urethane dimethacrylates improve conversion of dental composites and reveal complexity of cytocompatibility testing. Dent. Mater. 2016, 32, 264–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]










| Materials | Composition | Instructions |
|---|---|---|
| Formulation 1 (F1) | Powder: spherical pre-reacted glass fillers (SPG) (40 wt% irregular shape, 60% spherical shape) Liquid: CMH liquid; CM polymer (55 wt%), water (45 wt%), tartaric acid (2 pph), CQ (0.7 pph), DMAEMA (1.4 pph) |
|
| Formulation 2 (F2) | Powder: spherical pre-reacted glass fillers (SPG) (40 wt% irregular shape, 60% spherical shape) Liquid: CMH liquid; CM polymer (50 wt%), HEMA (5 wt%), water (45 wt%), tartaric acid (2 pph), CQ (0.7 pph), DMAEMA (1.4 pph) | |
| Vitrebond (VB) | Powder: glass powder (>95 wt%), diphenyliodonium chloride (<2 wt%) Liquid: copolymer of polyacids (35–45 wt%), HEMA (20–30 wt%), water (30–40 wt%) |
|
| TheraCal LC (TC) | Calcium silicate cement (30–50 wt%), polyethylene glycol dimethacrylate (10–30 wt%), barium zirconate powder (1–10 wt%) |
|
| Materials/Ion (Mean and SD, ppm) | Sr | Ca | Al | Na |
|---|---|---|---|---|
| F1 | 4.12 (0.15) | 1.85 (0.06) | 0.40 (0.04) | 0.77 (0.03) |
| F2 | 6.31 (0.09) | 3.08 (0.05) | 0.21 (0.01) | 0.58 (0.03) |
| VB | <0.1 * | <0.1 * | 0.26 (0.01) | 15.02 (0.21) |
| TC | 8.22 (0.19) | 26.91 (0.98) | 0.29 (0.01) | <0.01 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Potiprapanpong, W.; Thepveera, W.; Khamsuk, C.; Channasanon, S.; Tanodekaew, S.; Patntirapong, S.; Monmaturapoj, N.; Panpisut, P. Monomer Conversion, Dimensional Stability, Biaxial Flexural Strength, Ion Release, and Cytotoxicity of Resin-Modified Glass Ionomer Cements Containing Methacrylate-Functionalized Polyacids and Spherical Pre-Reacted Glass Fillers. Polymers 2021, 13, 2742. https://doi.org/10.3390/polym13162742
Potiprapanpong W, Thepveera W, Khamsuk C, Channasanon S, Tanodekaew S, Patntirapong S, Monmaturapoj N, Panpisut P. Monomer Conversion, Dimensional Stability, Biaxial Flexural Strength, Ion Release, and Cytotoxicity of Resin-Modified Glass Ionomer Cements Containing Methacrylate-Functionalized Polyacids and Spherical Pre-Reacted Glass Fillers. Polymers. 2021; 13(16):2742. https://doi.org/10.3390/polym13162742
Chicago/Turabian StylePotiprapanpong, Wisitsin, Whithipa Thepveera, Chutikarn Khamsuk, Somruethai Channasanon, Siriporn Tanodekaew, Somying Patntirapong, Naruporn Monmaturapoj, and Piyaphong Panpisut. 2021. "Monomer Conversion, Dimensional Stability, Biaxial Flexural Strength, Ion Release, and Cytotoxicity of Resin-Modified Glass Ionomer Cements Containing Methacrylate-Functionalized Polyacids and Spherical Pre-Reacted Glass Fillers" Polymers 13, no. 16: 2742. https://doi.org/10.3390/polym13162742