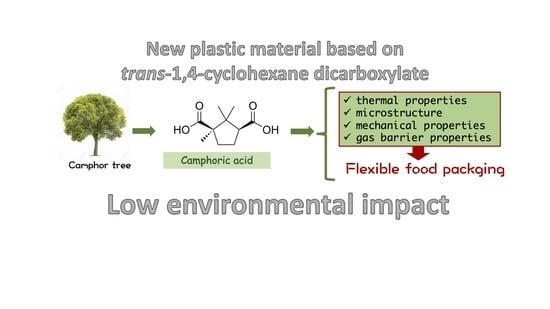
Chemical Modification of Poly(butylene trans-1,4-cyclohexanedicarboxylate) by Camphor: A New Example of Bio-Based Polyesters for Sustainable Food Packaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis
2.3. Molecular Characterization
2.4. Films by Compression Moulding
2.5. Thermal and Structural Characterization
2.6. Mechanical Characterization
2.7. Gass Barrier Properties Evaluation
3. Results and Discussion
3.1. Molecular Characterization
3.2. Thermal and Structural Characterization
3.3. Mechanical Characterization
3.4. Gas Barrier Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Plastics Europe. Plastics—The Facts 2020. An Analysis of European Plastics Production, Demand and Waste Data. Available online: https://www.plasticseurope.org/application/files/5715/1717/4180/Plastics_the_facts_2020_FINAL_for_website_one_page (accessed on 14 June 2021).
- McQuilken, K. Sustainable packaging and its role in the supply chain. Nutrac. World 2016, 19, 52–54. [Google Scholar]
- Larrañaga, A.; Lizundia, E. A review on the thermomechanical properties and biodegradation behaviour of polyesters. Eur. Polym. J. 2019, 121, 109296. [Google Scholar] [CrossRef]
- Ni, H.; Daum, J.L.; Thiltgen, P.R.; Soucek, M.D.; Simonsick, W.J., Jr.; Zhong, W.; Skaja, A.D. Cycloaliphatic polyester-based high-solids polyurethane coatings II. The effect of difunctional acid. Progr. Org. Coat. 2002, 45, 49–58. [Google Scholar] [CrossRef]
- Pilati, F.; Toselli, M.; Messori, M. Waterborne and Solvent Based Saturated Polyesters and Their End User Applications; Sanders, D., Ed.; Wiley: New York, NY, USA, 1999; (Chapter 2). [Google Scholar]
- Genovese, L.; Lotti, N.; Gazzano, M.; Finelli, L.; Munari, A. New eco-friendly random copolyesters based on poly (propylene cyclohexanedicarboxylate): Structure-properties relationships. Expr. Polym. Lett. 2015, 9, 972–983. [Google Scholar] [CrossRef]
- Genovese, L.; Soccio, M.; Gigli, M.; Lotti, N.; Gazzano, M.; Siracusa, V.; Munari, A. Gas permeability, mechanical behaviour and compostability of fully-aliphatic bio-based multiblock poly (ester urethane) s. RSC Adv. 2016, 6, 55331–55342. [Google Scholar] [CrossRef]
- Gigli, M.; Lotti, N.; Gazzano, M.; Siracusa, V.; Finelli, L.; Munari, A.; Dalla Rosa, M. Biodegradable aliphatic copolyesters containing PEG-like sequences for sustainable food packaging applications. Polym. Degrad. Stab. 2014, 105, 96–106. [Google Scholar] [CrossRef]
- Heidt, P.C.; Elliott, M.L. Aliphatic dibasic acid-modified polyesters thermoset industrial coatings. In Proceedings of the Waterborne, High-Solids, and Power Coatings Symposium, New Orleans, LA, USA, 23–25 February 1995. [Google Scholar]
- Berti, C.; Binassi, E.; Colonna, M.; Fiorini, M.; Kannan, G.; Karanam, S. Synthesis and radiocarbon evidence of terephthalate polyesters completely prepared from renewable resources. Green Chem. 2011, 13, 2543–2548. [Google Scholar]
- Gigli, M.; Lotti, N.; Gazzano, M.; Siracusa, V.; Finelli, L.; Munari, A.; Dalla Rosa, M. Fully aliphatic copolyesters based on poly (butylene 1,4- cyclohexanedicarboxylate) with promising mechanical and barrier properties for food packaging applications. Ind. Eng. Chem. Res. 2013, 52, 12876–12886. [Google Scholar] [CrossRef]
- Gigli, M.; Lotti, N.; Siracusa, V.; Gazzano, M.; Munari, A.; Dalla Rosa, M. Effect of molecular architecture and chemical structure on solid-state and barrier properties of heteroatom-containing aliphatic polyesters. Eur. Polym. J. 2016, 78, 314–325. [Google Scholar] [CrossRef]
- Siracusa, V.; Genovese, L.; Ingrao, C.; Munari, A.; Lotti, N. Barrier Properties of Poly (Propylene Cyclohexanedicarboxylate) Random Eco-Friendly Copolyesters. Polymers 2018, 10, 502. [Google Scholar] [CrossRef] [Green Version]
- Guidotti, G.; Soccio, M.; Siracusa, V.; Gazzano, M.; Munari, A.; Lotti, N. Novel Random Copolymers of Poly (butylene 1,4-cyclohexane dicarboxylate) with Outstanding Barrier Properties for Green and Sustainable Packaging: Content and Length of Aliphatic Side Chains as Efficient Tools to Tailor the Material’s Final Performance. Polymers 2018, 10, 866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, S.; Geng, Z.; Zhang, W.; Liang, J.; Wang, C.; Deng, Z.; Du, S. The Chemical Composition of Essential Oils from Cinnamomum camphora and Their Insecticidal Activity against the Stored Product Pests. Int. J. Mol. Sci. 2016, 17, 1836. [Google Scholar] [CrossRef] [PubMed]
- Talapatra, S.K.; Talapatra, B. Chemistry of Plant Natural Products; Springer: Berlin/Heidelberg, Germany; New Delhi, India, 2014; p. 377. [Google Scholar] [CrossRef]
- Hofer, M.; Müller, J. Fraunhofer IGB Press Release, Monomers from Camphor Enable Biobased Plastics; 3 August 2018. Available online: https://www.igb.fraunhofer.de/en/press-media/press-releases/2018/camphor-basedpolymers (accessed on 1 June 2021).
- Camphor as Viable Alternative to Castor Oil for Production Bio-PA? Available online: https://www.bioplasticsmagazine.com/en/news/meldungen/20181104-Can-camphor-offer-an-alternative-tocastor-oil-to-produce-bio-PA-.php (accessed on 3 May 2021).
- Chen, W.; Vermaak, I.; Viljoen, A. Camphor—A Fumigant during the Black Death and a Coveted Fragrant Wood in Ancient Egypt and Babylon—A Review. Molecules 2013, 18, 5434–5454. [Google Scholar] [CrossRef] [Green Version]
- Ritter, J.J. A New Camphor Synthesis. J. Am. Chem. Soc. 1933, 55, 3322–3326. Available online: https://pubs.acs.org/doi/pdf/10.1021/ja01335a0462002 (accessed on 3 May 2021). [CrossRef]
- Gubelmann, I.; Elley, H.W. American Production of Synthetic Camphor from Turpentine. Ind. Eng. Chem. 1934, 26, 589–594. [Google Scholar] [CrossRef]
- Beri, M.L.; Sarin, J.L. Production of synthetic camphor from Indian turpentine. J. Soc. Chem. Ind. 1936, 55, 605–607. [Google Scholar] [CrossRef]
- Zauba.com, Detailed Import Data of Camphor. Available online: https://www.zauba.com/import-camphorhs-code.html (accessed on 1 June 2021).
- O’Toole, S.E.; Rose, C.A.; Gundala, S.; Zeitler, K.; Connon, S.J. Highly Chemoselective Direct Crossed Aliphatic−Aromatic Acyloin Condensations with Triazolium-Derived Carbene Catalysts. J. Org. Chem. 2011, 76, 347–357. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Jiang, K.; Liu, T.-Y.; Chen, Y.-C. Asymmetric [4+1] Cycloadditions of N-Thioacylimines and Sulfur Ylides. Adv. Synth. Cat. 2017, 359, 2530–2534. [Google Scholar] [CrossRef]
- Xu, F.; Yan, L.; Lei, C.; Zhao, H.; Li, G. Asymmetric Cu-catalyzed Henry reaction promoted by chiral camphor-derived β-amino alcohols with a thiophene moiety. Tetr. Asym. 2015, 26, 338–343. [Google Scholar] [CrossRef]
- Wu, H.-L.; Wu, P.-Y.; Cheng, Y.-N.; Uang, B.-J. Enantioselective addition of organozinc reagents to carbonyl compounds catalyzed by a camphor derived chiral γ-amino thiol ligand. Tetrahedron 2016, 72, 2656–2665. [Google Scholar] [CrossRef]
- Scheirs, J.; Long, T.E. Modern Polyesters: Chemistry and Technology of Polyesters and Copolyesters Edited. In Wiley Series in Polymer Science; John Wiley & Sons, Ltd.: West Sussex, UK, 2003; Chapter 10; ISBN 978-0-471-49856-8. [Google Scholar]
- Toy, M.S. Stereoregular condensation polymers. I. Synthesis and comparison of optically active and inactive polycamphorates and polycamphoramides. J. Polym. Sci. Part A-1 Polym. Chem. 1967, 5, 2481–2486. [Google Scholar] [CrossRef]
- Zhang, H.; Li, J.; Tian, Z.; Liu, F. Synthesis and properties of novel alicyclic-functionalized polyimides prepared from natural—(D)-camphor. J. Appl. Polym. Sci. 2013, 129, 3333–3340. [Google Scholar] [CrossRef]
- Robert, C.; de Montigny, F.; Thomas, C.M. Tandem synthesis of alternating polyesters from renewable resources. Nat. Comm. 2011, 2, 586. [Google Scholar] [CrossRef]
- Fournier, L.; Robert, C.; Pourchet, S.; Gonzalez, A.; Williams, L.; Prunet, J.; Thomas, C.M. Facile and efficient chemical functionalization of aliphatic polyesters by cross metathesis. Polym. Chem. 2016, 7, 3700–3704. [Google Scholar] [CrossRef] [Green Version]
- Choi, G.-H.; Hwang, D.Y.; Suh, D.H. High Thermal Stability of Bio-Based Polycarbonates Containing Cyclic Ketal Moieties. Macromolecules 2015, 48, 6839–6845. [Google Scholar] [CrossRef]
- Park, J.E.; Hwang, D.Y.; Choi, G.H.; Choi, K.H.; Suh, D.H. Fast Hydrolysis Polyesters with a Rigid Cyclic Diol from Camphor. Biomacromolecules 2017, 18, 2633–2639. [Google Scholar] [CrossRef]
- Nsengiyumva, O.; Miller, S.A. Synthesis, characterization, and water-degradation of biorenewable polyesters derived from natural camphoric acid. Green Chem. 2019, 21, 973–978. [Google Scholar] [CrossRef]
- Pang, C.; Jiang, X.; Yu, Y.; Chen, L.; Ma, J.; Gao, H. Copolymerization of Natural Camphor-Derived Rigid Diol with Various Dicarboxylic Acids: Access to Biobased Polyesters with Various Properties. ACS Macro Lett. 2019, 8, 1442–1448. [Google Scholar] [CrossRef]
- Jiang, X.; Yan, Y.; Guan, Y.; Liu, T.; Pang, C.; Ma, J.; Gao, H. Random and Multiblock PBS Copolyesters Based on a Rigid Diol Derived from Naturally Occurring Camphor: Influence of Chemical Microstructure on Thermal and Mechanical Properties. ACS Sustain. Chem. Eng. 2020, 8, 3626–3636. [Google Scholar] [CrossRef]
- Anslyn, E.V.; Dougherty, D.A. Modern Physical Organic Chemistry: Strain and Stability; Murdzek, J., Ed.; University Science Book: Sausalito, CA, USA, 2005; Chapter 2; pp. 100–109. ISBN 1-891389-31-9. [Google Scholar]
- Stoclet, G.; Seguela, R.; Lefebvre, J.-M.; Rochas, C. New insights on the strain-induced mesophase of Poly (D,L-lactide): In situ WAXS and DSC study of the thermo-mechanical stability. Macromolecules 2010, 43, 7228–7237. [Google Scholar] [CrossRef]
- Lv, R.; Na, B.; Tian, N.; Zou, S.; Li, Z.; Jiang, S. Mesophase formation and its thermal transition in the stretched glassy polylactide revealed by infrared spectroscopy. Polymer 2011, 52, 4979–4984. [Google Scholar] [CrossRef]
- Guidotti, G.; Gigli, M.; Soccio, M.; Lotti, N.; Siracusa, V.; Munari, A. Poly (butylene 2,5-thiophenedicarboxylate): An Added Value to the Class of High Gas Barrier Biopolyesters. Polymers 2018, 10, 167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Genovese, L.; Gigli, M.; Lotti, N.; Gazzano, M.; Siracusa, V.; Munari, A.; Dalla Rosa, M. Biodegradable long chain aliphatic polyesters containing ether-linkages: Synthesis, solid-state, and barrier properties. Ind. Eng. Chem. Res. 2014, 53, 10965–10973. [Google Scholar] [CrossRef]
- Baur, H.; Baltorowicz, M. Influence of sequence-length. Distribution on the melting end point of copolymers. Makromol. Chem. 1966, 98, 297–301. [Google Scholar]
- Celli, A.; Marchese, P.; Sullalti, S.; Berti, C.; Barbiroli, G. Eco-friendly Poly (butylene 1,4-cyclohexanedicarboxylate): Relationships Between Stereochemistry and Crystallization Behavior. Macromol. Chem. Phys. 2011, 212, 1524–1534. [Google Scholar] [CrossRef]
- Guidotti, G.; Soccio, M.; Garcia-Gutiérrez, M.-C.; Gutierrez-Fernandez, E.; Ezquerra, T.A.; Siracusa, V.; Munari, A.; Lotti, N. Evidence of a 2D-Ordered Structure in Biobased Poly (pentamethylene furanoate) Responsible for Its Outstanding Barrier and Mechanical Properties. ACS Sustain. Chem. Eng. 2019, 7, 17863–17871. [Google Scholar] [CrossRef]
- Guidotti, G.; Soccio, M.; Garcia-Gutiérrez, M.-C.; Ezquerra, T.A.; Siracusa, V.; Gutierrez-Fernandez, E.; Munari, A.; Lotti, N. Fully Biobased Superpolymers of 2,5-Furandicarboxylic Acid with Different Functional Properties: From Rigid to Flexible, High Performant Packaging Materials. ACS Sustain. Chem. Eng. 2020, 8, 9558–9568. [Google Scholar] [CrossRef] [PubMed]
- Hedenqvist, M.S. Barrier packaging materials. In Environmental Degradation of Materials, 2nd ed.; Kutz, M., Ed.; William Andrew Publ.: Norwich, NY, USA, 2012; Chapter 26; pp. 547–563. [Google Scholar]
- Piergiovanni, L.; Limbo, L. Food Packaging; Springer: Milano, Italy, 2010; pp. 77–129. ISBN 978-88-470-1457-2. [Google Scholar]








| Polymer | BCE mol% (1H-NMR) | Cis-Isomer mol% (1H-NMR) | Mn g/mol | D |
|---|---|---|---|---|
| PBCE | 100 | 4 | 41,000 | 1.4 |
| P(BCE95BCE5) | 95 | 6 | 48,300 | 1.5 |
| P(BCE90BC10) | 91 | 7 | 24,500 | 1.7 |
| P(BCE85BC15) | 84 | 8 | 28,200 | 1.5 |
| Polymer | Tonset °C | Tmax °C | I SCAN Powders | I SCAN Films | II SCAN | Xc Powders % | Xc Films % | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tm °C | ΔHm J/g | Tm °C | ΔHm J/g | Tg °C | Δcp J/g·°C | Tm °C | ΔHm J/g | |||||
| PBCE | 395 | 418 | 54 | 1.94 | 53 | 1.32 | 16 | 0.124 | 166 | 29 | 24 | 23 |
| 167 | 40 | 166 | 30 | |||||||||
| P(BCE95BC5) | 392 | 417 | 50 | 1.98 | 47 | 1.37 | 12 | 0.141 | 153 | 22 | 9 | 20 |
| 152 | 32 | 153 | 26 | |||||||||
| P(BCE90BC10) | 391 | 417 | 42 | 1.19 | 47 | 1.54 | 8 | 0.173 | 141 | 19 | 9 | 21 |
| 141 | 25 | 140 | 21 | |||||||||
| P(BCE85BC15) | 385 | 416 | 44 | 1.11 | 47 | 1.88 | 7 | 0.173 | 132 | 18 | 5 | 18 |
| 130 | 24 | 131 | 20 | |||||||||
| Polymer | E (MPa) | σY (MPa) | εY (%) | σB (MPa) | εB (%) | GTR-O2 (cm3cm/m2 d atm) | GTR-CO2 (cm3cm/m2 d atm) |
|---|---|---|---|---|---|---|---|
| PBCE | 560 ± 19 | - | - | 27 ± 2 | 33 ± 5 | 2.54 | 5.34 |
| P(BCE95BC5) | 492 ± 31 | 24 ± 1 | 24 ± 6 | 22 ± 4 | 250 ± 30 | 0.93 | 2.54 |
| P(BCE90BC10) | 391 ± 24 | - | - | 18 ± 2 | 20 ± 3 | 1.84 | 4.61 |
| P(BCE85BC15) | 326 ± 21 | - | - | 17 ± 3 | 22 ± 4 | 2.86 | 5.83 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guidotti, G.; Burzotta, G.; Soccio, M.; Gazzano, M.; Siracusa, V.; Munari, A.; Lotti, N. Chemical Modification of Poly(butylene trans-1,4-cyclohexanedicarboxylate) by Camphor: A New Example of Bio-Based Polyesters for Sustainable Food Packaging. Polymers 2021, 13, 2707. https://doi.org/10.3390/polym13162707
Guidotti G, Burzotta G, Soccio M, Gazzano M, Siracusa V, Munari A, Lotti N. Chemical Modification of Poly(butylene trans-1,4-cyclohexanedicarboxylate) by Camphor: A New Example of Bio-Based Polyesters for Sustainable Food Packaging. Polymers. 2021; 13(16):2707. https://doi.org/10.3390/polym13162707
Chicago/Turabian StyleGuidotti, Giulia, Gianfranco Burzotta, Michelina Soccio, Massimo Gazzano, Valentina Siracusa, Andrea Munari, and Nadia Lotti. 2021. "Chemical Modification of Poly(butylene trans-1,4-cyclohexanedicarboxylate) by Camphor: A New Example of Bio-Based Polyesters for Sustainable Food Packaging" Polymers 13, no. 16: 2707. https://doi.org/10.3390/polym13162707