Fluoropolymer/Glycidyl Azide Polymer (GAP) Block Copolyurethane as New Energetic Binders: Synthesis, Mechanical Properties, and Thermal Performance
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Polymerization of PBFMO
2.3. Synthesis of PBFMO-b-GAP Copolyurethanes
2.4. Characterization
3. Result and Discussion
3.1. Preparation of PBFMO-b-GAP Copolyurethanes
3.2. Density, Sensitivity, and XPS of PBFMO-b-GAP Copolyurethanes
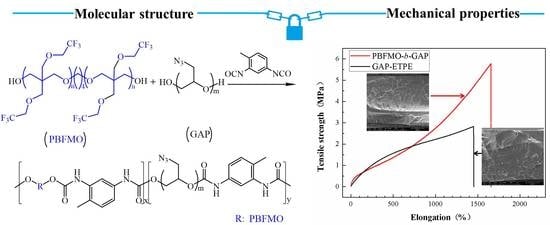
3.3. Mechanical Properties of PBFMO-b-GAP Copolyurethanes
3.4. Thermal Decomposition
3.5. Compatibility Testing
3.6. Cook-Off Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheng, T. Review of novel energetic polymers and binders-high energy propellant ingredients for the new space race. Des. Monomers Polym. 2019, 22, 54–65. [Google Scholar] [CrossRef] [Green Version]
- Bodaghi, A.; Shahidzadeh, M. Synthesis and characterization of new PGN based reactive oligomeric plasticizers for glycidyl azide polymer. Propellants Explos. Pyrotech. 2018, 43, 364–370. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, L.; Zhang, X.; Mi, Z. Thermal stability and kinetic of decomposition of nitrated HTPB. J. Hazard. Mater. 2009, 172, 1659–1664. [Google Scholar] [CrossRef]
- Hafner, S.; Keicher, T.; Klapoetke, T.M. Copolymers based on GAP and 1,2-epoxyhexane as promising prepolymers for energetic binder systems. Propellants Explos. Pyrotech. 2018, 43, 126–135. [Google Scholar] [CrossRef]
- Boopathi, S.K.; Hadjichristidis, N.; Gnanou, Y.; Feng, X. Direct access to poly(glycidyl azide) and its copolymers through anionic (co-)polymerization of glycidyl azide. Nat. Commun. 2019, 10, 293–301. [Google Scholar] [CrossRef] [Green Version]
- Frankel, M.B.; Grant, L.R.; Flanagan, J.E. Historical development of glycidyl azide polymer. J. Propuls. Power. 1992, 8, 560–563. [Google Scholar] [CrossRef]
- Murali Mohan, Y.; Mani, Y.; Mohana Raju, K. Synthesis of azido polymers as potential energetic propellent binders. Des. Monomers Polym. 2006, 9, 201–236. [Google Scholar] [CrossRef]
- Selim, K.; Ozkar, S.; Yilmaz, L. Thermal characterization of glycidyl azide polymer (GAP) and GAP-based binders for composite propellants. J. Appl. Polym. Sci. 2000, 77, 538–546. [Google Scholar] [CrossRef]
- Gaur, B.; Lochab, B.; Choudhary, V.; Varma, I.K. Azido polymers-Energetic binders for solid rocket propellants. J. Macromol. Sci. Polym. Rev. 2003, 43, 505–545. [Google Scholar] [CrossRef]
- Ding, Y.; Hu, C.; Guo, X.; Che, Y.; Huang, J. Structure and mechanical properties of novel composites based on glycidyl azide polymer and propargyl-terminated polybutadiene as potential binder of solid propellant. J. Appl. Polym. Sci. 2014, 131, 40007–40014. [Google Scholar] [CrossRef]
- Li, Y.J.; Ma, S.; Deng, J.K.; Luo, Y.J. Study on bulk preparation and properties of glycidyl azide polymer with hydroxyl–terminated polyether elastomers obtained through step-wise curing process. Colloid. Polym. Sci. 2017, 295, 637–646. [Google Scholar] [CrossRef]
- Li, P.; Li, Q.; Li, X.L.; Gan, X.X.; Yu, H.J. Research on the mechanical property of GAP copolymer elastomer. Chin. J. Explos. Propellants 2000, 23, 23–28. [Google Scholar]
- Sikder, A.K.; Reddy, S. Review on energetic thermoplastic elastomers (ETPEs) for military science. Propellants Explos. Pyrotech. 2013, 38, 14–28. [Google Scholar] [CrossRef]
- Yanagisawa, Y.; Nan, Y.; Okuro, K.; Aida, T. Mechanically robust, readily repairable polymers via tailored noncovalent cross-linking. Science 2018, 359, 72–80. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Jian, X.; Xiao, L.; Zhou, W. Microphase separation and mechanical performance of thermoplastic elastomers based on poly(glycidyl azide)/poly(oxytetramethylene glycol). Polym. Eng. Sci. 2018, 58, 167–173. [Google Scholar] [CrossRef]
- Wang, G.; Luo, Y. Characterization of P(BAMO/AMMO) ETPE prepared using different diisocyanates. Propellants Explos. Pyrotech. 2016, 41, 850–854. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, G.; Wang, Z.; Zhang, Y.; Ge, Z.; Luo, Y. Synthesis and characterization of novel energetic thermoplastic elastomers based on glycidyl azide polymer (GAP) with bonding functions. Polym. Bull. 2015, 72, 1835–1847. [Google Scholar] [CrossRef]
- Lee, I.; Reed, R.R.; Brady, V.L.; Finnegan, S.A. Energy release in the reaction of metal powders with fluorine containing polymers. J. Therm. Anal. 1997, 49, 1699–1705. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, S.J.; Park, J.S.; Kim, J.H. Energetic Al/Fe2O3/PVDF composites for high energy release: Importance of polymer binder and interface. Macromol. Res. 2016, 24, 909–914. [Google Scholar] [CrossRef]
- Dattelbaum, D.M.; Sheffield, S.A.; Stahl, D.; Weinberg, M.; Neel, C.; Thadhani, N. Equation of state and high pressure properties of a fluorinated terpolymer: THV 500. J. Appl. Phys. 2008, 104, 113525–113535. [Google Scholar] [CrossRef]
- McCollum, J.; Pantoya, M.L.; Iacono, S.T. Activating aluminum reactivity with fluoropolymer coatings for improved energetic composite combustion. ACS Appl. Mater. Interfaces 2015, 7, 18742–18749. [Google Scholar] [CrossRef]
- Gong, F.; Guo, H.; Zhang, J.; Shen, C.; Lin, C.; Zeng, C.; Liu, S. Highly thermal stable TATB–based aluminized explosives realizing optimized balance between thermal stability and detonation performance. Propellants Explos. Pyrotech. 2017, 42, 1424–1430. [Google Scholar] [CrossRef]
- Yang, H.; Huang, C.; Chen, H. Tuning reactivity of nanoaluminum with fluoropolymer via electrospray deposition. J. Therm. Anal. Calorim. 2017, 127, 2293–2299. [Google Scholar] [CrossRef]
- Rider, K.B.; Little, B.K.; Emery, S.B.; Lindsay, C.M. Thermal analysis of magnesium/perfluoropolyether pyrolants. Propellants Explos. Pyrotech. 2013, 38, 433–440. [Google Scholar] [CrossRef]
- Jiang, W.C.; Huang, Y.G.; Gu, G.T.; Meng, W.D.; Qing, F.L. A novel waterborne polyurethane containing short fluoroalkyl chains: Synthesis, characterization and its application on cotton fabrics surface. Appl. Surf. Sci. 2006, 253, 2304–2309. [Google Scholar] [CrossRef]
- Ma, S.; Li, Y.; Li, Y.J.; Li, G.P.; Luo, Y.J. Research on the mechanical properties and curing networks of energetic GAP/TDI binders. Cent. Eur. J. Energetic Mater. 2017, 14, 708–725. [Google Scholar] [CrossRef]
- Wang, X.; Hu, J.; Li, Y.; Zhang, J.; Ding, Y. The surface properties and corrosion resistance of fluorinated polyurethane coatings. J. Fluor. Chem. 2015, 176, 14–19. [Google Scholar] [CrossRef]
- Liu, X.; Gao, H.; Chen, X.; Hu, Y.; Pei, S.; Li, H.; Zhang, Y. Synthesis of perfluorinated ionomers and their anion exchange membranes. J. Membr. Sci. 2016, 515, 268–276. [Google Scholar] [CrossRef]
- Tanver, A.; Rehman, F.; Wazir, A.; Khalid, S.; Ma, S.; Li, X.; Luo, Y.; Huang, M.H. Energetic hybrid polymer network (EHPN) through facile sequential polyurethane curation based on the reactivity differences between glycidyl azide polymer and hydroxyl terminated polybutadiene. RSC Adv. 2016, 6, 11032–11039. [Google Scholar] [CrossRef]
- Ma, M.; Kwon, Y. Reactive cycloalkane plasticizers covalently linked to energetic polyurethane binders via facile control of an in situ Cu-free azide-alkyne 1,3-dipolar cycloaddition reaction. Polym. Chem. 2018, 9, 5452–5461. [Google Scholar] [CrossRef]
- Jin, B.; Shen, J.; Gou, X.; Peng, R.; Chu, S.; Dong, H. Synthesis, characterization, thermal stability and sensitivity properties of new energetic polymers-PVTNP-g-GAPs crosslinked polymers. Polymers 2016, 8, 10. [Google Scholar] [CrossRef] [Green Version]
- Shmatova, O.I.; Nenajdenko, V.G. Tetrazole-substituted five, six, and seven-membered cyclic amines bearing perfluoroalkyl groups efficient synthesis by azido-ugi reaction. Eur. J. Org. Chem. 2013, 28, 6397–6403. [Google Scholar] [CrossRef]
- Sarangapani, R.; Reddy, S.T.; Sikder, A.K. Molecular dynamics simulations to calculate glass transition temperature and elastic constants of novel polyethers. J. Mol. Graph. Modell. 2015, 57, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.F.; Jin, B.; Peng, R.F.; Zhang, Q.C.; Gong, W.L.; Huang, H.J.; Chu, S.J.; Dong, H.S. Synthesis, spectroscopic characterization, thermal stability and compatibility properties of energetic PVB-g-GAP copolymers. J. Polym. Res. 2015, 22, 167–177. [Google Scholar] [CrossRef]
- Cai, T.; Yang, W.J.; Neoh, K.G.; Kang, E.T. Poly(vinylidene fluoride) membranes with hyperbranched antifouling and antibacterial polymer brushes. Ind. Eng. Chem. Res. 2012, 51, 15962–15973. [Google Scholar] [CrossRef]
- Sangermano, M.; Bongiovanni, R.; Malucelli, G.; Priola, A.; Pollicino, A.; Recca, A. Fluorinated epoxides as surface modifying agents of UV curable systems. Appl. Polym. Sci. 2003, 89, 1524–1529. [Google Scholar] [CrossRef]
- Xiong, J.S.; Jin, Y.Q.; Shentu, B.Q.; Weng, Z.X. Preparation and fluorine enrichment behavior of fluorinated polyester. J. Coat. Technol. Res. 2013, 10, 621–629. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, T.F.; Zhao, B.B.; Luo, Y.J. Effect of nitrocellulose (NC) on morphology, rheological and mechanical properties of glycidyl azide polymer based energetic thermoplastic elastomer/NC blends. Polym. Int. 2017, 66, 705–711. [Google Scholar] [CrossRef]
- Xu, M.H.; Ge, Z.X.; Lu, X.M.; Mo, H.C.; Ji, Y.P.; Hu, H.M. Structure and mechanical properties of fluorine-containing glycidyl azide polymer-based energetic binders. Polym. Int. 2017, 66, 1318–1323. [Google Scholar] [CrossRef]
- Lee, W.J.; Cha, S.H. Improvement of mechanical and self–healing properties for polymethacrylate derivatives containing maleimide modified graphene oxide. Polymers 2020, 12, 603. [Google Scholar] [CrossRef] [Green Version]
- Zirnstein, B.; Schulze, D.; Schartel, B. Mechanical and fire properties of multicomponent flame retardant EPDM rubbers using aluminum trihydroxide, ammonium polyphosphate, and polyaniline. Materials 2019, 12, 1932. [Google Scholar] [CrossRef] [Green Version]
- Malkappa, K.; Jana, T. Simultaneous improvement of tensile strength and elongation: An unprecedented observation in the case of hydroxyl terminated polybutadiene polyurethanes. Ind. Eng. Chem. Res. 2013, 36, 12887–12896. [Google Scholar] [CrossRef]
- Landsem, E.; Jensen, T.L.; Kristensen, T.E.; Hansen, F.K.; Benneche, T.; Unneberg, E. Isocyanate-free and dual curing of smokeless composite rocket propellants. Propellants Explos. Pyrotech. 2013, 38, 75–86. [Google Scholar] [CrossRef]
- You, J.S.; Kweon, J.O.; Kang, S.C.; Noh, S.T. A kinetic study of thermal decomposition of glycidyl azide polymer (GAP)-based energetic thermoplastic polyurethanes. Macromol. Res. 2010, 18, 1226–1232. [Google Scholar] [CrossRef]
- Pisharath, S.; Ang, H.G. Synthesis and thermal decomposition of GAP–Poly(BAMO) copolymer. Polym. Degra. Stabil. 2007, 92, 1365–1377. [Google Scholar] [CrossRef]
- Guo, M.L.; Ma, Z.L.; He, L.M.; He, W.; Wang, Y.W. Effect of varied proportion of GAP-ETPE/NC as binder on thermal decomposition behaviors, stability and mechanical properties of nitramine propellants. J. Therm. Anal. Calorim. 2017, 130, 909–918. [Google Scholar] [CrossRef]
- Wang, G.; Ge, Z.; Luo, Y.J. Thermal decomposition kinetics of poly(3,30-bisazidomethyl oxetane-3-azidomethyl-30-methyl oxetane). J. Therm. Anal. Calorim. 2015, 122, 1515–1523. [Google Scholar] [CrossRef]
- Pei, J.F.; Zhao, F.Q.; Lu, H.L.; Song, X.D.; Zhou, R.; Yuan, Z.F.; Zhang, J.; Chen, J.B. Compatibility study of BAMO-GAP copolymer with some energetic materials. J. Therm. Anal. Calorim. 2016, 124, 1301–1307. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Ma, S.; Luo, Y. Compatibility, mechanical and thermal properties of GAP/P(EO-co-THF) blends obtained upon a urethane-curing reaction. Polym. Bull. 2017, 74, 4607–4618. [Google Scholar] [CrossRef]
- Ding, X.Y.; Shu, Y.J.; Xu, H.T.; Chen, Z.Q. Study on thermal behaviour of AP/LiBH4 energetic system. Propellants Explos. Pyrotech. 2018, 43, 267–273. [Google Scholar] [CrossRef]
- Li, W.F.; Yu, Y.G.; Ye, R.; Yang, H.W. Three-dimensional simulation of base bleed unit with AP/HTPB propellant in fast cook–off conditions. J. Energetic Mater. 2017, 35, 265–275. [Google Scholar] [CrossRef]
- Chen, L.; Ma, X.; Lu, F.; Wu, J.Y. Investigation of the cook-off processes of HMX-based mixed explosives. Cent. Eur. J. Energetic Mat. 2014, 11, 199–218. [Google Scholar]
- Xu, M.H.; Ge, Z.X.; Lu, X.M.; Mo, H.C.; Ji, Y.P.; Hu, H.M. Fluorinated glycidyl azide polymers as potential energetic binders. RSC Adv. 2017, 7, 47271–47278. [Google Scholar] [CrossRef] [Green Version]












| Sample | PBFMO/GAP Molar Ratio | Theoretical Content of F Element | Mn (103 gmol−1) | Density (g cm−3) | H50 (cm) |
|---|---|---|---|---|---|
| PBFMO-b-GAP-1# | 1/3 | 10.1 | 33 | 1.308 | >129 |
| PBFMO-b-GAP-2# | 1/9 | 4.04 | 31 | 1.290 | >129 |
| PBFMO-b-GAP-3# | 1/19 | 2.02 | 30 | 1.273 | 56.2 |
| GAP-ETPE | 0 | 0 | 32 | 1.263 | 9.55 |
| Sample | C (%) | O (%) | N (%) | F (%) |
|---|---|---|---|---|
| PBFMO-b-GAP-1# | 65.22 | 20.18 | 1.54 | 13.05 |
| PBFMO-b-GAP-2# | 63.00 | 19.76 | 4.33 | 12.91 |
| PBFMO-b-GAP-3# | 60.88 | 23.42 | 10.36 | 5.34 |
| GAP-ETPE | 63.8 | 24.04 | 12.16 | 0 |
| Scheme | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|
| PBFMO-b-GAP-1# | 5.75 ± 0.275 | 1660 ± 42.3 |
| PBFMO-b-GAP-2# | 3.65 ± 0.13 | 1874 ± 58 |
| PBFMO-b-GAP-3# | 2.9 ± 0.11 | 2056 ± 47.3 |
| GAP-ETPE | 2.81 ± 0.124 | 1446 ± 52 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, M.; Lu, X.; Liu, N.; Zhang, Q.; Mo, H.; Ge, Z. Fluoropolymer/Glycidyl Azide Polymer (GAP) Block Copolyurethane as New Energetic Binders: Synthesis, Mechanical Properties, and Thermal Performance. Polymers 2021, 13, 2706. https://doi.org/10.3390/polym13162706
Xu M, Lu X, Liu N, Zhang Q, Mo H, Ge Z. Fluoropolymer/Glycidyl Azide Polymer (GAP) Block Copolyurethane as New Energetic Binders: Synthesis, Mechanical Properties, and Thermal Performance. Polymers. 2021; 13(16):2706. https://doi.org/10.3390/polym13162706
Chicago/Turabian StyleXu, Minghui, Xianming Lu, Ning Liu, Qian Zhang, Hongchang Mo, and Zhongxue Ge. 2021. "Fluoropolymer/Glycidyl Azide Polymer (GAP) Block Copolyurethane as New Energetic Binders: Synthesis, Mechanical Properties, and Thermal Performance" Polymers 13, no. 16: 2706. https://doi.org/10.3390/polym13162706