1. Introduction
Ultraviolet (UV)-initiated photopolymerization systems have been fast-growing and widely used in many applications where the traditional thermal curing system has been almost impossible for decades, because the polymerizations can be carried out solvent-free at room temperature [
1,
2,
3,
4,
5]. UV curing technology has the advantages of fast curing speed, less pollution and energy efficiency and ambient temperature requirement [
6,
7,
8]. UV-curable technology is applied extensively in the printing industry, biomedical applications, adhesives, 3D printing and the information industry [
9,
10,
11,
12].
UV curing technology mainly has two curing mechanisms, free radical curing [
13,
14] and cationic curing [
15,
16,
17]. The free radical curing system has developed a variety of prepolymer monomers, which has the advantages of fast reaction speed and easy adjustment of the cured film performance. However, the shortcomings such as large volume shrinkage and poor adhesion restrict its development [
18,
19,
20,
21]. The advantages of cationic photopolymerization are wear resistance, high hardness, small volume shrinkage, strong adhesion [
22,
23,
24]. The biggest advantage of cationic photopolymerization is that it does not exhibit oxygen inhibition like radical-based systems do [
25,
26,
27,
28].
With the rapid development of UV-curing technology, cationic UV-curing systems are attracting increased attention because of their outstanding advantages. Cycloaliphatic epoxides, oxetanes and bisphenol A type epoxy compound are used as prepolymers in the cationic curing systems [
29,
30]. Compared to the cycloaliphatic epoxides of cationic UV-curing systems, oxetane polymerization have a lower viscosity, lower toxicity, higher curing rate and higher thermal stability [
30,
31]. However, the photosensitivity of oxetanes is worse than the cycloaliphatic epoxides, which limits its scope of application for oxetanes [
32,
33,
34,
35].
Since Rhone Poulenc synthesized UV-curable silicone-modified acrylate in 1979, more and more people have begun to study the subject of combining UV-curable resins with silicone materials [
36]. Scholars have introduced silicone materials into UV-curable resins, and can have advantages such as high weather resistance, low surface tension, low viscosity and low Tg of silicone materials to improve the weather resistance [
37], thermal stability [
38] and hardness [
39] of UV-curable materials, also giving the coating better stain resistance [
40]. However, such a modification has been accomplished by the addition of silicone benzene materials with oxetanes systems that, to the best of our knowledge, is presented for the first time in literature. It can increase the competitiveness of UV-curable materials in the field of 3D printing.
In this paper, diethyl carbonate and trihydroxypropane were used to synthesize the precursor EHO(3-ethyl-3-hydroxymethyloxetane); the precursor and allyl bromide as raw materials were used to generate the intermediate AllylEHO(3-ethyl-3-allylmethoxyoxetane); and finally prepolymer bis[(3-ethyl-3-methoxyoxetane)propyl]diphenylsilane was synthesized by AllylEHO and diphenylsilane. A series of photosensitive resin products were prepared with photoinitiator triarylsulfonium hexafluoroantimonate of 3% and prepolymer, which were subjected to Photo-DSC test, TG test, mechanical performance test and light transmittance.
2. Experimental
2.1. Materials
Diethyl carbonate (99.7%), trihydroxypropane (99.7%), anhydrous magnesium sulfate were purchased from Sinopharm Chemical Reagent Co., Ltd., Shanghai, China. Potassium hydroxide, allyl bromide (99.7%) were purchased from Tianjin Damao Chemical Reagent Factory, Tianjin, China. Dichloromethane (99.7%) and tetrabutylammonium bromide (99.7%) were purchased from Xilong Chemical Co., Ltd., Guangdong, China. Toluene (99.9%) was purchased from Nanjing Runsheng Petrochemical Co., Ltd., Nanjing, China. Cationic photoinitiator triarylsulfonium hexafluoroantimonate (99.7%) was purchased from Dow Union Carbide, namely, diphenylsilane (99.7%) was purchased from Beijing Bailingwei Technology Co., Ltd., Beijing, China. Tris(triphenylphosphine) rhodium chloride (99.7%) was purchased from Shanghai Yishi Chemical Co., Ltd., Beijing, China. All of them are analytically pure. E-51 (98%) was purchased from Shanghai Resin Factory, 3,4-Epoxycyclohexylmethyl-3′,4′- epoxy cyclohexane carboxylate (2021P) (98%) was purchased from Daicel (China) Investment Co., Ltd., Shanghai, China.
2.2. Characterization Methods
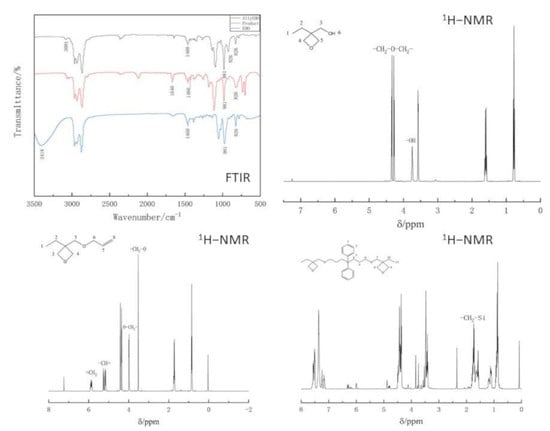
1H-NMR spectra of synthesized oxetanes have been recorded on a 400MR DD2 Nuclear Magnetic Resonance Spectrometer (American Agilent Technologies Co., Ltd., Palo Alto, CA, USA), operating at 600 MHz using CDCl3 as a solvent and Tetramethylsilane (TMS) as an internal reference. The FTIR spectra have been recorded on Nicolet 5700 Smart Fourier Transformation Red External spectrometer (American Thermoelectric Nicolas Corporation., MA, USA), in a transmittance mode, in the range of 4000–400 cm−1 at a resolution of 4 cm−1. Photo-DSC measurements have been taken with a TA Instruments Q2000 Photo-DSC apparatus (TA Instruments, New Castle, PA, USA) equipped with a mercury lamp and UV-intensity of 35 mW/cm2, under the nitrogen atmosphere (flow rate was 50 mL/min), using aluminum sample pans. Thermogravimetric analysis (TGA) has been performed with TGA 4000 Thermogravimetric Analyzer (American PE Company., Waltham, MA, USA), in a range of 25 to 800 °C at a heating rate of 10 °C/min in a stream of nitrogen (60 mL/min). Mechanical properties have been taken with CMT4204 microcomputer-controlled universal material testing machine (Shenzhen New Sansi Company, Shenzhen, China) at room temperature. Light transmittance has been performed with CARY 100 UV-Vis Spectrophotometer (Agilent Technologies Co., Ltd., California, CA, USA) in ranges of 240–800 nm (chloroform).
2.3. Synthesis
2.3.1. Synthesis of 3-Ethyl-3-hydroxymethyloxetane Monomer
The weighed diethyl carbonate and trimethylolpropane were added to a 250 mL three-necked flask equipped with a thermometer, a stir bar, and a reflux condenser, and then an appropriate amount of methanol solvent and potassium hydroxide catalyst was added to the three-necked flask. Reflux reaction maintained for 1h at the temperature of 115 °C until the transesterification reaction of diethyl carbonate and trihydroxypropane had been completed and the intermediate of six-membered ring lactone structure was obtained. The reflux device was then converted into a distillation device, and the products produced by the reaction were collected by distillation. Then the reaction system was heated to 205–210 °C to remove CO
2, vacuum distillation collected 130~135 °C/21.3 kPa fraction, and the collected fraction was the desired target product 3-ethyl-3-hydroxymethyloxetane. The synthesis path is shown in
Figure 1.
2.3.2. Synthesis of 3-Ethyl-3-allylmethoxyoxetane
The distilled fraction 3-ethyl-3-hydroxymethyloxetane and 50% KOH aqueous solution were added to a three-necked flask equipped with a magnetic stirrer, a thermometer, a separatory funnel and a reflux tube. Tetrabutylammonium bromide was added to the mixture with vigorously stirring and allyl bromide in separatory funnel were added to the three-necked flask at a rate of one drop per second and stirred at 0 °C for 24 h. An appropriate amount of dichloromethane solution and distilled water were added to the reaction product and a separatory funnel was used to separate the phases. Organic phase was washed twice with distilled water, dried and filtered through anhydrous magnesium sulfate, and excess allyl bromide and extractant dichloromethane was removed by using a rotary evaporator. The residue was distilled under reduced pressure to collect 55–60 °C /0.4 kPa fraction, the product obtained is 3-ethyl-3-allylmethoxyoxetane (AllylEHO). The synthesis path is shown in
Figure 2.
2.3.3. Synthesis of Bis[(3-ethyl-3-methoxyoxetane)propyl]diphenylsilane
An appropriate amount of toluene,3-ethyl-3-allylmethoxyoxetane and tris(triphenylphosphine)rhodium chloride catalyst were accurately weighed into a 500 mL three-necked round bottom flask equipped with thermometer and condenser and a dropping funnel. The mixture of toluene and diphenylsilane in the dropping funnel was added into the flask while the temperature of mixture solution was kept at 88–92 °C, maintaining a titration rate of about 1.5 s for one drop. After the titration, the temperature was kept at 88–92 °C for 4–5 h until the reaction was completed. It would undergo a hydrosilylation reaction to obtain bis[(3-ethyl-3-methoxyoxetane)propyl]diphenylsilane. Then, under a vacuum condition, the mixture was heated to 100 °C, the solvent was distilled with a rotary evaporator to obtain the reacted product. The synthesis path is shown in
Figure 3.
2.4. The Preparation of UV-Cured Spline
The prepolymer bis[(3-ethyl-3-methoxyoxetane)propyl]diphenylsilane and photoinitiator triarylsulfonium hexafluoroantimonate of 3% were mixed well, standing for a period of time until there were no bubbles in the mixed solution. The mixed solution was poured into the spline mold and evenly smeared the mixed solution on the glass sheets, putted them into the intelligent control light curing machine, and the light intensity was adjusted to 90% and cured for 60 s to obtain the UV-cured splines and films.
4. Conclusions
3-ethyl-3-hydroxymethyloxetane was synthesized by diethyl carbonate and trihydroxypropane in an alkaline environment; 3-ethyl-3-allylmethoxyoxetane was synthesized by allyl bromide and 3-ethyl-3-hydroxymethyloxetane; bis[(3-ethyl-3-methoxyoxetane)propyl]diphenylsilane was synthesized by 3-ethyl-3-allylmethoxyoxetane and diphenylsilane. Triarylsulfonium hexafluoroantimonate of 3% was added to bis[(3-ethyl-3-methoxyoxetane)propyl]diphenylsilane to prepare a new type of cationic photosensitive resin. Such a modification has been accomplished by the polymerization of oxetane with silicon benzene compound, which is presented for the first time in the literature, to the best of our knowledge.
The realization of such an approach has been confirmed using 1H-NMR, and FTIR spectroscopies. The photosensitivity has been determined by using photo-DSC, the introduction of diphenylsilane has improved the photosensitivity of oxtane. Besides, UV-cured product of bis[(3-ethyl-3-methoxyoxetane)propyl]diphenylsilane has great mechanical properties and thermal properties determined TG analyses and mechanical tests, because of the introduction of phenyl and silicon atoms. The light transmittance remains above 98%. Excellent photosensitivity of bis[(3-ethyl-3-methoxyoxetane)propyl]diphenylsilane meets the needs of 3D printing for resin materials, which further broadens the preparation range of UV-curable products, and has certain promotion and application value.