3D-Printable Hierarchical Nanogel-GelMA Composite Hydrogel System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Gelatin Methacryloyl (GelMA)
2.3. 1H-NMR of Gelatin and GelMA
2.4. Synthesis of Core-shell Nanogel (Amine-NG)
2.5. Synthesis of Methacryloyl-Functionalized Nanogel (MA-NG)
2.6. Transmission Electron Microscopy (TEM) of Nanogels
2.7. Dynamic Light Scattering and Zeta Potential Measurements of Nanogels
2.8. Potentiometric Titration of Nanogels
2.9. Preparation of MA-NG-GelMA Composite Hydrogels and Scanning Electron Microscopy (SEM) Analyses
2.10. Printability in 3D of MA-NG-GelMA and Confocal Laser Scanning Microscopy
2.11. Fluorescence Spectroscopy of MA-NG-GelMA and Amine-NG-GelMA Composite Hydrogels
3. Results and Discussion
3.1. Synthesis and Characterization of GelMA

3.2. Synthesis and Characterization of Nanogels
3.3. Preparation and Morphology of MA-NG-GelMA Composite Hydrogel
3.4. Covalent Bonding of MA-NG within MA-NG-GelMA Hydrogels
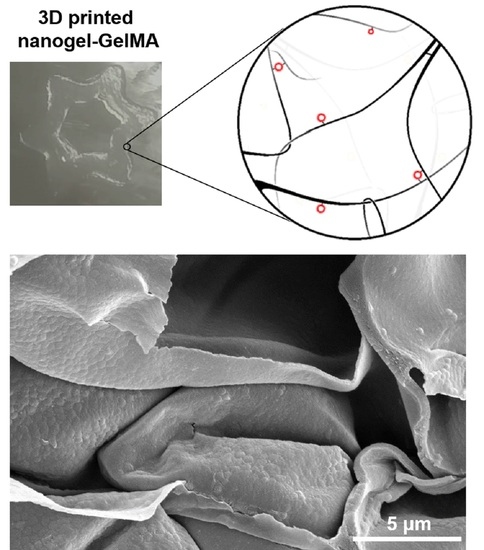
3.5. Printability in 3D of MA-NG-GelMA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haycock, J.W. 3D cell culture: A review of current approaches and techniques. Methods Mol. Biol. 2011, 695, 1–15. [Google Scholar]
- Fontoura, J.C.; Viezzer, C.; dos Santos, F.G.; Ligabue, R.A.; Weinlich, R.; Puga, R.D.; Antonow, D.; Severino, P.; Bonorino, C. Comparison of 2D and 3D cell culture models for cell growth, gene expression and drug resistance. Mater. Sci. Eng. C 2020, 107, 110264. [Google Scholar] [CrossRef]
- Ravi, M.; Paramesh, V.; Kaviya, S.R.; Anuradha, E.; Solomon, F.D.P. 3D cell culture systems: Advantages and applications. J. Cell. Physiol. 2015, 230, 16–26. [Google Scholar] [CrossRef]
- Klotz, B.J.; Gawlitta, D.; Rosenberg, A.J.W.P.; Malda, J.; Melchels, F.P.W. Gelatin-methacryloyl hydrogels: Towards biofabrication-based tissue repair. Trends Biotechnol. 2016, 34, 394–407. [Google Scholar] [CrossRef] [Green Version]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef]
- Jang, J.; Park, J.Y.; Gao, G.; Cho, D.W. Biomaterials-based 3D cell printing for next-generation therapeutics and diagnostics. Biomaterials 2018, 156, 88–106. [Google Scholar] [CrossRef]
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef] [Green Version]
- Yin, J.; Yan, M.; Wang, Y.; Fu, J.; Suo, H. 3D bioprinting of low-concentration cell-laden gelatin methacrylate (GelMA) bioinks with a two-step cross-linking strategy. ACS Appl. Mater. Interfaces 2018, 10, 6849–6857. [Google Scholar] [CrossRef]
- Pepelanova, I.; Kruppa, K.; Scheper, T.; Lavrentieva, A. Gelatin-methacryloyl (GelMA) hydrogels with defined degree of functionalization as a versatile toolkit for 3D cell culture and extrusion bioprinting. Bioengineering 2018, 5, 55. [Google Scholar] [CrossRef] [Green Version]
- Gao, Q.; Niu, X.; Shao, L.; Zhou, L.; Lin, Z.; Sun, A.; Fu, J.; Chen, Z.; Hu, J.; Liu, Y.; et al. 3D printing of complex GelMA-based scaffolds with nanoclay. Biofabrication 2019, 11, 035006. [Google Scholar] [CrossRef]
- Liu, Y.; Chan-Park, M.B. A biomimetic hydrogel based on methacrylated dextran-graft-lysine and gelatin for 3D smooth muscle cell culture. Biomaterials 2010, 31, 1158–1170. [Google Scholar] [CrossRef]
- Nichol, J.W.; Koshy, S.T.; Bae, H.; Hwang, C.M.; Yamanlar, S.; Khademhosseini, A. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials 2010, 31, 5536–5544. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.R.; Yong, K.W.; Choi, J.Y.; Cowie, A.C. Recent advances in photo-crosslinkable hydrogels for biomedical applications. Biotechniques 2019, 66, 40–53. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.-H.; Su, J.J.-M.; Lee, S.-Y.; Lin, Y.-M. Stiffness modification of photopolymerizable gelatin-methacrylate hydrogels influences endothelial differentiation of human mesenchymal stem cells. J. Tissue Eng. Regen. Med. 2018, 12, 2099–2111. [Google Scholar] [CrossRef]
- Ratcliffe, J.H.; Hunneyball, I.M.; Smith, A.; Wilson, C.G.; Davis, S.S. Preparation and evaluation of biodegradable polymeric systems for the intra-articular delivery of drugs. J. Pharm. Pharmacol. 1984, 36, 431–436. [Google Scholar] [CrossRef]
- Dubruel, P.; Unger, R.; Van Vlierberghe, S.; Cnudde, V.; Jacobs, P.J.S.; Schacht, E.; Kirkpatrick, C.J. Porous gelatin hydrogels: 2. In vitro cell interaction study. Biomacromolecules 2007, 8, 338–344. [Google Scholar] [CrossRef]
- Lin, R.Z.; Chen, Y.C.; Moreno-Luna, R.; Khademhosseini, A.; Melero-Martin, J.M. Transdermal regulation of vascular network bioengineering using aphotopolymerizable methacrylated gelatin hydrogel. Biomaterials 2013, 34, 6785–6796. [Google Scholar] [CrossRef] [Green Version]
- Jeon, O.; Wolfson, D.W.; Alsberg, E. In-situ formation of growth-factor-loaded coacervate microparticle-embedded hydrogels for directing encapsulated stem cell fate. Adv. Mater. 2015, 27, 2216–2223. [Google Scholar] [CrossRef]
- Grogan, S.P.; Chung, P.H.; Soman, P.; Chen, P.; Lotz, M.K.; Chen, S.; D’Lima, D.D. Digital micromirror device projection printing system for meniscus tissue engineering. Acta Biomater. 2013, 9, 7218–7226. [Google Scholar] [CrossRef] [Green Version]
- Mahadik, B.P.; Pedron Haba, S.; Skertich, L.J.; Harley, B.A.C. The use of covalently immobilized stem cell factor to selectively affect hematopoietic stem cell activity within a gelatin hydrogel. Biomaterials 2015, 67, 297–307. [Google Scholar] [CrossRef] [Green Version]
- Sivakumaran, D.; Maitland, D.; Hoare, T. Injectable microgel-hydrogel composites for prolonged small-molecule drug delivery. Biomacromolecules 2011, 12, 4112–4120. [Google Scholar] [CrossRef]
- Elkhoury, K.; Sanchez-Gonzalez, L.; Lavrador, P.; Almeida, R.; Gaspar, V.; Kahn, C.; Cleymand, F.; Arab-Tehrany, E.; Mano, J.F. Gelatin methacryloyl (GelMA) nanocomposite hydrogels embedding bioactive naringin liposomes. Polymers 2020, 12, 2944. [Google Scholar] [CrossRef]
- Keskin, D.; Zu, G.; Forson, A.M.; Tromp, L.; Sjollema, J.; van Rijn, P. Nanogels: A novel approach in antimicrobial delivery systems and antimicrobial coatings. Bioact. Mater. 2021, 6, 3634–3657. [Google Scholar] [CrossRef]
- Yu, K.; Yang, X.; He, L.; Zheng, R.; Min, J.; Su, H.; Shan, S.; Jia, Q. Facile preparation of pH/reduction dual-stimuli responsive dextran nanogel as environment-sensitive carrier of doxorubicin. Polymer 2020, 200, 122585. [Google Scholar] [CrossRef]
- Molina, M.; Asadian-Birjand, M.; Balach, J.; Bergueiro, J.; Miceli, E.; Calderón, M. Stimuli-responsive nanogel composites and their application in nanomedicine. Chem. Soc. Rev. 2015, 44, 6161–6186. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Z.; Chen, J.; Cui, L.; Zhuang, X.; Ding, J.; Chen, X. Advances in stimuli-responsive polypeptide nanogels. Small Methods 2018, 2, 1700307. [Google Scholar] [CrossRef]
- Mergel, O.; Schneider, S.; Tiwari, R.; Kühn, P.T.; Keskin, D.; Stuart, M.C.A.; Schöttner, S.; De Kanter, M.; Noyong, M.; Caumanns, T.; et al. Cargo shuttling by electrochemical switching of core-shell microgels obtained by a facile one-shot polymerization. Chem. Sci. 2019, 10, 1844–1856. [Google Scholar] [CrossRef] [Green Version]
- Zu, G.; Steinmüller, M.; Keskin, D.; Van Der Mei, H.C.; Mergel, O.; Van Rijn, P. Antimicrobial nanogels with nanoinjection capabilities for delivery of the hydrophobic antibacterial agent triclosan. ACS Appl. Polym. Mater. 2020, 2, 5779–5789. [Google Scholar] [CrossRef]
- Zu, G.; Mergel, O.; Ribovski, L.; Bron, R.; Zuhorn, I.S.; van Rijn, P. Nanogels with selective intracellular reactivity for intracellular tracking and delivery. Chem. A Eur. J. 2020, 26, 15084–15088. [Google Scholar]
- Keskin, D.; Tromp, L.; Mergel, O.; Zu, G.; Warszawik, E.; Van Der Mei, H.C.; Van Rijn, P. Highly efficient antimicrobial and antifouling surface coatings with triclosan-loaded nanogels. ACS Appl. Mater. Interfaces 2020, 12, 57721–57731. [Google Scholar] [CrossRef]
- Brosel-Oliu, S.; Mergel, O.; Uria, N.; Abramova, N.; Van Rijn, P.; Bratov, A. 3D impedimetric sensors as a tool for monitoring bacterial response to antibiotics. Lab Chip 2019, 19, 1436–1447. [Google Scholar] [CrossRef] [Green Version]
- Loessner, D.; Meinert, C.; Kaemmerer, E.; Martine, L.C.; Yue, K.; Levett, P.A.; Klein, T.J.; Melchels, F.P.W.; Khademhosseini, A.; Hutmacher, D.W. Functionalization, preparation and use of cell-laden gelatin methacryloyl-based hydrogels as modular tissue culture platforms. Nat. Protoc. 2016, 11, 727–746. [Google Scholar] [CrossRef] [Green Version]
- Gelissen, A.P.H.; Schmid, A.J.; Plamper, F.A.; Pergushov, D.V.; Richtering, W. Quaternized microgels as soft templates for polyelectrolyte layer-by-layer assemblies. Polymer 2014, 55, 1991–1999. [Google Scholar] [CrossRef]
- Schuurman, W.; Levett, P.A.; Pot, M.W.; van Weeren, P.R.; Dhert, W.J.A.; Hutmacher, D.W.; Melchels, F.P.W.; Klein, T.J.; Malda, J. Gelatin-methacrylamide hydrogels as potential biomaterials for fabrication of tissue-engineered cartilage constructs. Macromol. Biosci. 2013, 13, 551–561. [Google Scholar] [CrossRef]
- Shin, H.; Olsen, B.D.; Khademhosseini, A. The mechanical properties and cytotoxicity of cell-laden double-network hydrogels based on photocrosslinkable gelatin and gellan gum biomacromolecules. Biomaterials 2012, 33, 3143–3152. [Google Scholar] [CrossRef] [Green Version]
- Fairbanks, B.D.; Schwartz, M.P.; Bowman, C.N.; Anseth, K.S. Photoinitiated polymerization of PEG-diacrylate with lithium phenyl-2,4,6-trimethylbenzoylphosphinate: Polymerization rate and cytocompatibility. Biomaterials 2009, 30, 6702–6707. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.-C.; Lin, R.-Z.; Qi, H.; Yang, Y.; Bae, H.; Melero-Martin, J.M.; Khademhosseini, A. Functional human vascular network generated in photocrosslinkable gelatin methacrylate hydrogels. Adv. Funct. Mater. 2012, 22, 2027–2039. [Google Scholar] [CrossRef] [Green Version]
- Rinoldi, C.; Lanzi, M.; Fiorelli, R.; Nakielski, P.; Zembrzycki, K.; Kowalewski, T.; Urbanek, O.; Grippo, V.; Jezierska-Woźniak, K.; Maksymowicz, W.; et al. Three-Dimensional Printable Conductive Semi-Interpenetrating Polymer Network Hydrogel for Neural Tissue Applications. Biomacromolecules 2021, 22, 3084–3098. [Google Scholar] [CrossRef]







| Component | Chemical | Abbreviation | Mass (mg) | Molar Amount (mmol) | Molar Content (%) |
|---|---|---|---|---|---|
| Core | Monomer | NIPAM | 1505 | 13.3 | 95 |
| Cross-linker | BIS | 108 | 0.7 | 5 | |
| Surfactant | CTAB | 4 | 0.011 | - | |
| Dye | MRB | 10 | 0.015 | - | |
| Initiator | AMPA V50 | 54 | 0.2 | - | |
| Shell | Monomer | NIPAM | 673 | 5.95 | 85 |
| Comonomer | APMA | 125 | 0.7 | 10 | |
| Cross-linker | BIS | 54 | 0.35 | 5 | |
| Surfactant | CTAB | 2 | 0.005 | - | |
| Dye | MRB | 5 | 0.008 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zu, G.; Meijer, M.; Mergel, O.; Zhang, H.; van Rijn, P. 3D-Printable Hierarchical Nanogel-GelMA Composite Hydrogel System. Polymers 2021, 13, 2508. https://doi.org/10.3390/polym13152508
Zu G, Meijer M, Mergel O, Zhang H, van Rijn P. 3D-Printable Hierarchical Nanogel-GelMA Composite Hydrogel System. Polymers. 2021; 13(15):2508. https://doi.org/10.3390/polym13152508
Chicago/Turabian StyleZu, Guangyue, Marnix Meijer, Olga Mergel, Heng Zhang, and Patrick van Rijn. 2021. "3D-Printable Hierarchical Nanogel-GelMA Composite Hydrogel System" Polymers 13, no. 15: 2508. https://doi.org/10.3390/polym13152508